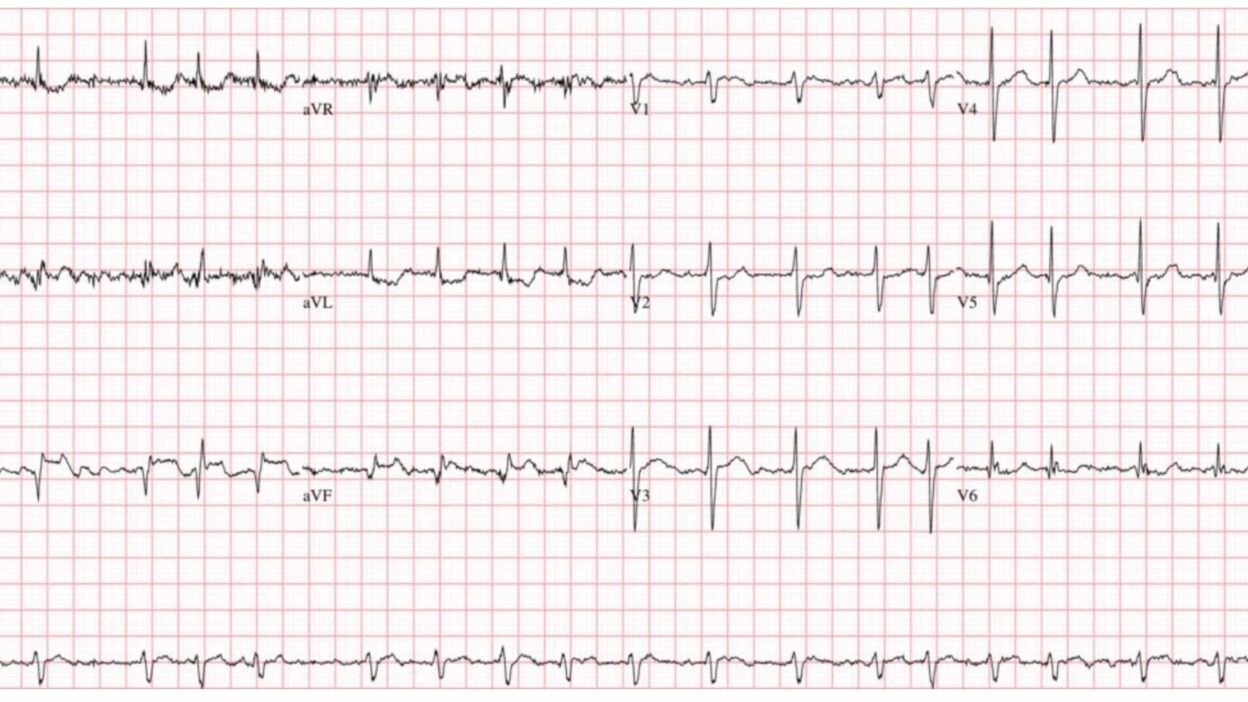
A 55-year-old woman presented to the ED complaining of “severe indigestion” with abdominal pain, nausea, and vomiting. Given her active vomiting and trembling in triage— this ECG was the best quality tracing that could be recorded.
What’s the diagnosis? (See below)
Watch this video breaking down this very important ECG case and see what happened!
Smith: this is an easy ECG; it is a STEMI(+) OMI and the patient needs the cath lab now. However, it does not appear to be the ECG of an acute MI; rather, the ECG is suggestive of a SUBACUTE MI. Why? There are Q-waves present, which, though that may happen acutely in anterior MI, it does not happen within the first hours of inferior MI. Moreover, the T-waves are flat compared to the ST segments. A truly acute MI has T-waves that are comparatively larger than the ST segments are elevated. This low acuteness of the ECG is consistent with the history of several hours of symptoms. Alternatively, it is also consistent with an acute MI superimposed on an old MI.
You can read all about “Acuteness” on the ECG here.
The main lesson to be learned here is that many MI do not present with chest discomfort. In fact, 1/3 of STEMI and 1/3 of NSTEMI do not have chest pain. Reference: Canto JG, Shlipak MG, Roger WJ. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000;283(24):3223–9.
Thus, patients with discomfort anywhere from the epigastrium to the lower face and upper arms/shoulders require an ECG. The interpretation of the ECG can be affected by the pretest probability. The pretest prob for chest discomfort is far higher than for epigastric pain or jaw pain, for instance. For epigastric discomfort it is lower, and the ECG needs to be more specific in order to make the diagnosis.
This is not a silent MI. Silent MI is different: there are no ACS symptoms at all, and the MI is only discovered later by a routine ECG showing Q-waves, then confirmed with imaging. Or perhaps it is discovered by other cardiac imaging done at a later time. It is estimated that one third of all MI are silent. Many more are missed (symptoms present, but the doctor misses the diagnosis).
======================================
MY Comment, by KEN GRAUER, MD (12/1/2025):
Today’s ECG Video Case is loaded with important Learning Points. I’ll add the following thoughts to the excellent video case discussion by Dr. Ghali.
- PEARL #1: As emphasized by Dr. Ghali — today’s patient had an extensive acute MI despite not having chest pain. As I reviewed in My Comment in the December 6, 2022 post — the entity of “Silent” MI occurs much more frequently than many clinicians appreciate (ie, in up to 30% of all MIs — patients either have no symptoms at all or — present with symptoms other than chest pain, such as nausea, vomiting and “indigestion”, as in today’s case).
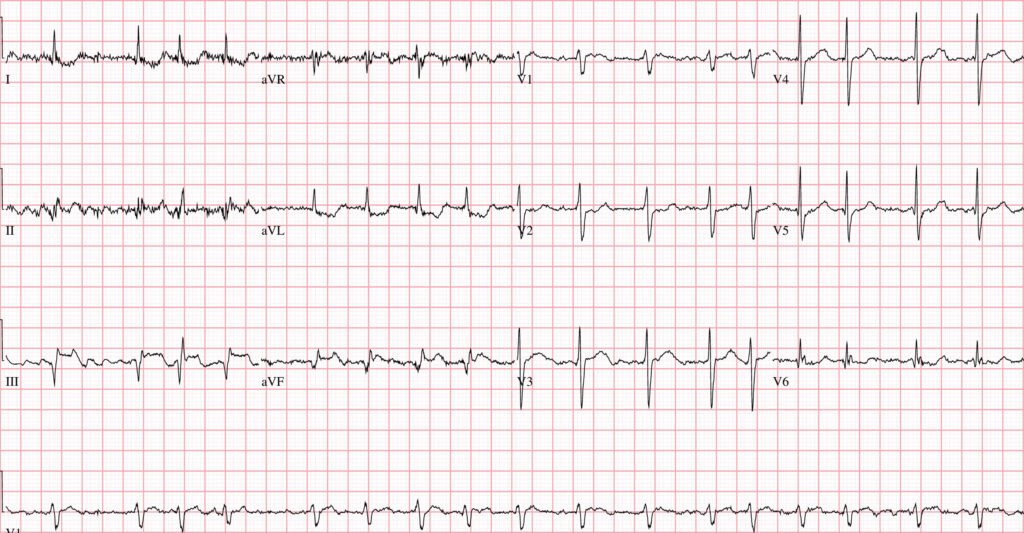
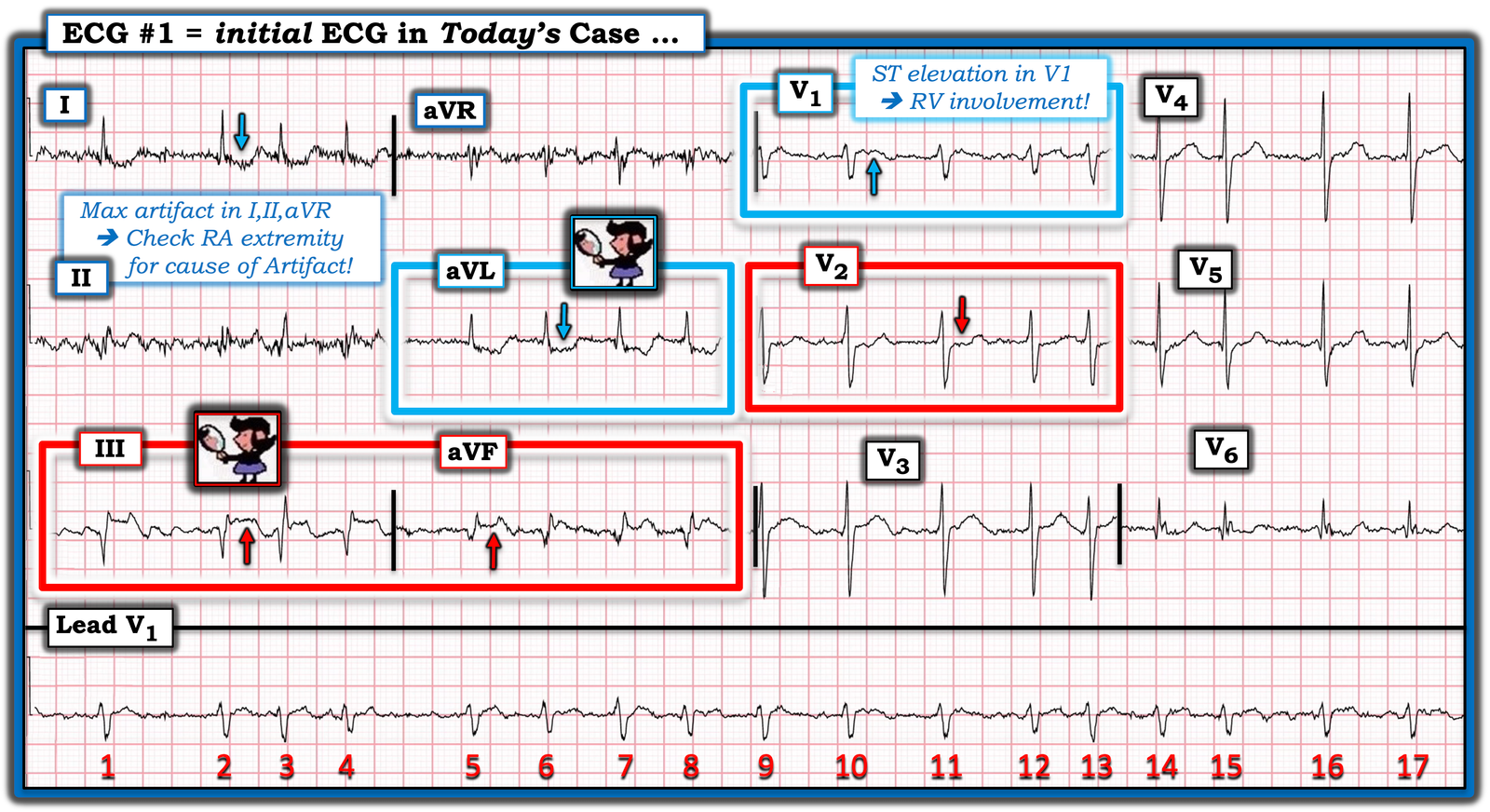
- PEARL #2: Today’s initial ECG is remarkable for the presence of ST elevation in leads III and aVF (within the RED rectangle for these leads in Figure-1). As per Dr. Ghali — the diagnosis of acute inferior OMI is strengthened by the finding of reciprocal ST depression in lead aVL. It is this “magic” mirror-image opposite ST-T wave picture that we see in leads III and aVL that distinguishes the inferior lead ST elevation of acute MI from repolarization variants or tachycardia (as fast heart rates sometimes produce a surprising amount of ST elevation that disappears once the rate slows).
- PEARL #3: Acute inferior MI generally results from acute RCA occlusion or LCx occlusion (with RCA occlusion being most common given that ~85% of people have a dominant RCA vessel). The finding in Figure-1 of marked ST elevation in lead III that is greater than the amount of ST elevation in lead II supports a RCA “culprit” artery.
- PEARL #4: Although Dr. Ghali emphasized the diagnosis of acute MI in today’s case exclusively from the limb leads — I find input from the chest leads to be of invaluable diagnostic assistance, especially in cases for which I might not be certain about an OMI from only the limb leads. Most inferior OMIs are associated with acute posterior OMI (as there usually is a common blood supply to both inferior and posterior walls of the left ventricle). Therefore — if you are at all uncertain about whether or not there is an inferior OMI — IF you see clear evidence of posterior OMI — then you know that an acute OMI is present.
- PEARL #5: The KEY to recognizing acute posterior OMI — is appreciation of ST segment flattening or depression, with loss of the usual slight upsloping ST elevation that is normally seen in both leads V2 and V3. As soon as I saw the ST flattening and depression highlighted by the RED arrow in lead V2 — I knew with 100% certainty that: i) There is a posterior OMI; — and, ii) Seeing this posterior OMI confirms the inferior OMI. Total time to recognize the posterior OMI in today’s case (once you condition yourself to routinely assess the ST-T wave in leads V2,V3 in all cases of suspected inferior OMI) = less than 3 seconds.
= = =
- PEARL #6: Normally there is at most minimal ST coving/elevation in lead V1. This is especially true when there is acute posterior OMI — which we’d expect to produce ST depression in lead V1. The fact that there is definite ST elevation in lead V1 (BLUE arrow in this lead) and — no more than minimal ST depression in lead V2 — suggests that something must be attenuating the amount of ST depression that we would otherwise expect to see in the anterior leads with posterior OMI. This tells us that in addition to acute infero-postero OMI — there is also acute RV involvement (ie, lead V1 is a right-sided lead that sometimes shows ST elevation with acute RV MI).
- PEARL #7: We could confirm acute RV MI by obtaining another ECG with right-sided leads. Awareness of acute RV involvement is especially important in today’s case given potential hemodynamic consequences of this patient’s AFib (See PEARL #9 below).
- PEARL #8: Knowing that there is acute RCA occlusion with RV involvement — localizes the site of occlusion to the proximal RCA (which is precisely what the cath film shows in Dr. Ghali’s video presentation).
= = =
Figure-1: I’ve labeled today’s ECG.
PEARL #9: Today’s patient presented with AFib and a moderately rapid ventricular response. That said — we are not told if her AFib is a new or longstanding arrhythmia — nor are we told if she remained in AFib at the time of her discharge from the hospital.
- It’s important to appreciate that especially if this patient’s AFib is new and precipitated by her acute MI — that new-onset AFib in the setting of an acute MI is a poor prognostic sign, with potential for predisposing to adverse hemodynamic effects through tachycardia (that reduces LV filling time) — loss of the “atrial kick” — and, AV dyssynchrony — each of which may contribute to reduce cardiac output, that in the setting of an acute MI could predispose to cardiogenic shock (Obayashi et al — JAMA 10(18), 2025).
- The overall incidence of new AFib during the early hours of acute MI is between 4-10% (Obayashi et al). Fortunately, new AFib in this setting is often transient when there is timely reperfusion.
PEARL #10: We can tell at a glance that although today’s patient was tremulous and actively vomiting (therefore with contribution to the artifact on this tracing from anywhere in her body) — the prime “culprit extremity” producing artifact is her RA (Right Arm extremity). We can deduce this because the greatest amount of artifact is seen in leads I, II and aVR (ie, electrical potential for leads I and II both use the RA electrode, whereas lead III does not — and artifact is greatest in augmented lead aVR, and less in leads aVL and aVF).
- NOTE: See My Comment at the bottom of the page in the December 5, 2022 post for review on determining the “culprit” extremity. (For links to over 50 examples of other “Technical Misadventures” — including many cases of artifact — CLICK HERE ).
- The clinical importance of knowing where to look for the cause of artifact — is that accurate ECG diagnosis of the acute OMI in today’s case depends on obtaining the best possible ECG recording (and knowing that the predominant cause of artifact in the limb leads is coming from movement in the RA extremity — may expedite recognizing potential corrective measures that might minimize this artifact).
= = =
= = =