An 89-year-old woman with a history of hypertension only on propranolol and hydrochlorothiazide presented by ambulance with sudden onset of chest pain. It radiated to her back. Medics reported that there was a slight difference in blood pressure between the 2 arms.
They recorded a prehospital ECG:
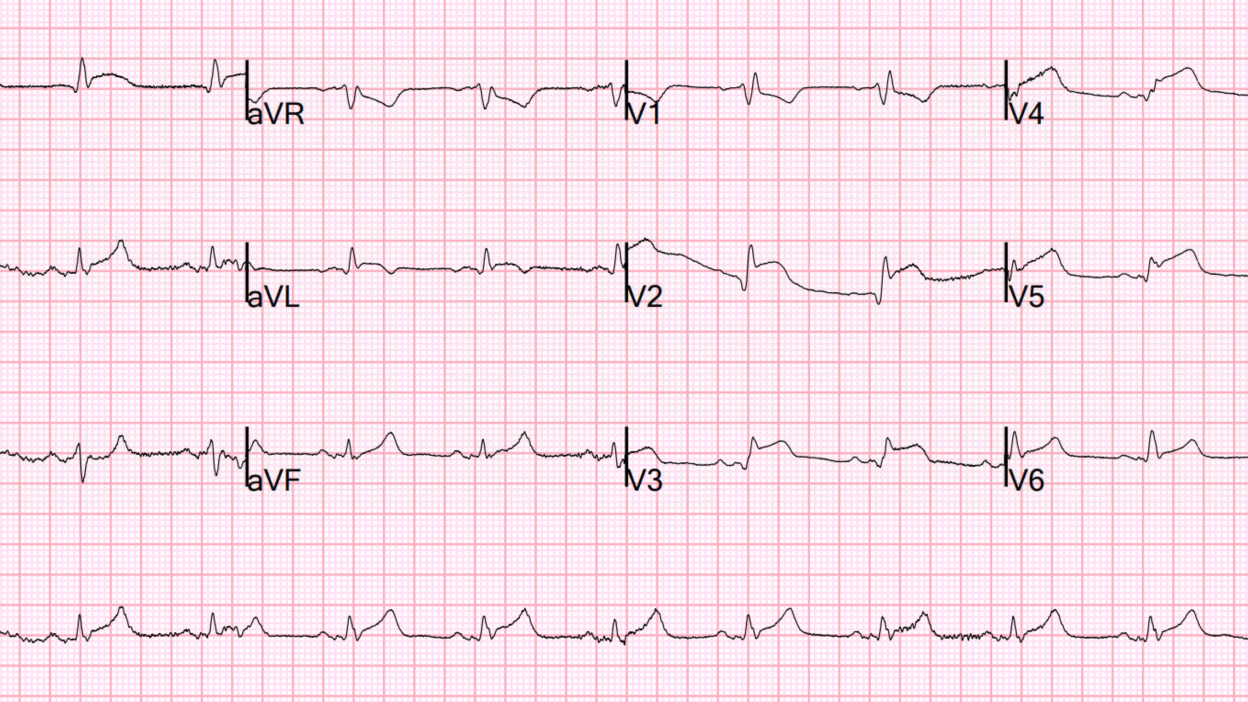
What do you think?
It shows RBBB with OMI in V2-V6, I, aVL and reciprocal STD in inferior leads, diagnostic of proximal LAD occlusion. This is an easy ECG.
On arrival she was in quite a bit of distress from pain. Vital signs were fine. I confirmed her ECG findings and activated the Cath Lab. Because of the radiation to the back and the unequal blood pressures we considered aortic dissection but at the same time did not want to delay treatment for this deadly myocardial infarction in order to do a workup for aortic dissection. If this was dissection, she would have no chance of survival.
I checked her left radial pulse and it was strong.
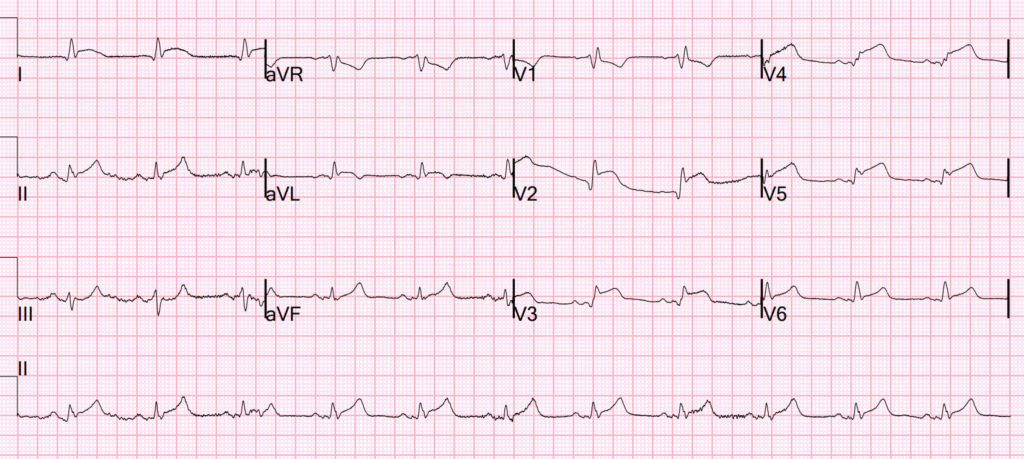
We recorded and ED ECG which was not significantly different:
Bedside cardiac echo showed a dense anterior wall motion abnormality.
The Cath Lab was occupied and so we needed to arrange a transfer to another PCI capable Hospital. While arranging the transfer, we gave heparin and ticagrelor. We gave her morphine. She was awake and alert with a good blood pressure of 130/80.
Only approximately 1% of “STEMI” (the subset of OMI which meet STEMI mm critieria) are due to dissection. In general, to take the time to get a CT is detrimental to patient care.
Instead, use bedside ultrasound to look for dissection (learn how to perform this exam!) This study showed very high sensitivity and specificity of bedside ultrasound for Aortic dissection.
So while we were waiting for transport, we had enough time to look at her aorta with ultrasound.

Parasternal long axis: The white arrow points to what we thought was a flap in the aortic root. (The yellow arrows show the anterior and posterior extent of the aortic root.
We then used the suprasternal notch as a window to view the aortic arch:

There appear to be 2 lumens, with a flap between them.
I did not think she could survive an aortic dissection down her left main coronary with huge anterior OMI. I was not 100% certain I was correct about the ultrasound. I decided that we needed to go to the cath lab because her only chance of survival was if it was a standard ACS OMI (due to ruptured plaque, not due to aortic dissection) and for the occlusion to be stented.
Just as we are transferring her to the ambulance cart, she became unresponsive. On the monitor she had electrical activity.
So this was PEA.
We did an immediate ultrasound which showed a massive hemopericardium with tamponade. We realized this was not survivable. But we did go through with some resuscitation because she continued to blink and reach for the sky and appeared to be conscious.
On next look with the ultrasound, the hemopericardium had disappeared! We then looked with ultrasound at her left chest to assess for hemothorax and there was indeed a massive hemothorax, indicating that the hemopericardium had ruptured freely into the chest.
Now she had exsanguinated and we realized there was absolutely no chance of survival and we declared death and stopped resuscitation.
= = =
======================================
MY Comment, by KEN GRAUER, MD (9/10/2025):
Today’s insightful case by Dr. Smith illustrates the difficult decision-making process inherent in situations in which the differential diagnosis is between two equally life-threatening emergencies.
- As per Dr. Smith — the history of new-onset severe CP (Chest Pain) in this 89-year old woman with RBBB and diffuse ST elevation on ECG was diagnostic of an acute extensive LAD OMI.
- Yet the history of CP radiating to the back — in association with unequal upper extremity pulses in this elderly woman with known hypertension — was all-but-diagnostic of a Type A Aortic Dissection.
- Patients with acute Type A Aortic Dissection (ie, dissection of the ascending aorta) generally do not survive without immediate surgical repair. But delay to obtain a diagnostic CT scan might prove equally fatal given clear ECG evidence of extensive ongoing anterolateral STEMI.
- Realistically — IF the cause of this 89-year old woman’s acute anterolateral STEMI was a Type A Aortic Dissection, her chance of survival was virtually nil regardless of any potential intervention. The decision-to-be-made was difficult but clear — the patient’s only realistic chance of survival would be to treat her acute MI with prompt cath and PCI, and hope that she did not have an acute dissection.
- Unfortunately — the patient’s condition abruptly deteriorated. Not surprisingly — she could not be resuscitated.
Teaching Points:
An excellent review on diagnosis and management of Type A Aortic Dissection by Acharya and Mariscalco can be found in Br J Cardiol 30:62-68, 2023. In addition, I found discussion of a dissection case in the December 16, 2024 post in Dr. Smith’s ECG Blog especially insightful.
- To Emphasize: As unfortunate as the end result of today’s case was — this outcome was inevitable. The path to this patient’s only realistic chance for survival was taken — but her extensive anterolateral STEMI resulting from acute Type A Aortic Dissection was simply not treatable.
= = =
By way of review — I’ve excerpted below a series of Teaching Points from Dr. Smith’s discussion in the above-cited December 16, 2024 post.
- Acute decision-making can be extremely difficult when confronted with the clinical reality of having to decide about whether to delay treatment of an acute OMI in order to get a CT scan to rule out acute aortic dissection. As a Result — IF the clinical setting, the history and the ECG all strongly suggest an acute OMI — it will usually not be worthwhile to delay cardiac cath and PCI (thereby risking additional myocardial loss) by getting a CT scan. Pre-Test probability is important!
- Acute Type A Aortic Dissection is rare. Acute OMI is much more common. Pre-Test probability is important.
- Statistically — Most dissection that causes OMI occurs in the RCA (usually near the ostium = the takeoff of the RCA). Today’s case did not follow this statistical generality — in that the acute STEMI was the result of acute LAD occlusion.
- Only ~1% of acute STEMIs are the result of aortic dissection.
- Only ~5% of aortic dissections cause an acute OMI.
- Pay Attention when the history and exam suggest possible aortic dissection (ie, in the older hypertensive patient with CP radiating to the back — especially when there is asymmetry between left and right upper and/or lower extremity pulses).
- Get an Ultrasound in the ED! Unlike the delay inherent in obtaining a CT scan — bedside ultrasound by the emergency physician can be done very quickly, and in skilled hands, is surprisingly sensitive and specific for acute aortic dissection (As shown above in Dr. Smith’s discussion — as well as in the impressive illustrations in the December 16, 2024 post).