Written by Jesse McLaren, with comments from Smith
A 75 year old with a previous MI and ischemic cardiomyopathy presented with 6/10 chest and epigastric pain ongoing for 4 hours. Serial ECGs were ‘STEMI negative’ but revealed an acute RBBB. Here are the serial precordial leads: what’s going on?
Triage ECG: “ST depression” or reciprocal change?
Here’s the baseline ECG and full triage ECG, with final interpretation. What do you think?
Baseline ECG:
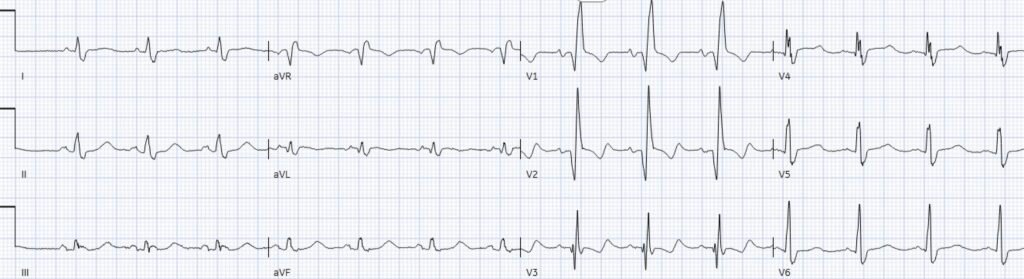
Triage ECG:
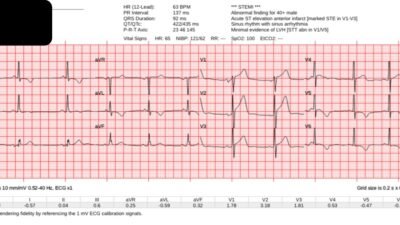
There’s normal sinus rhythm and high lead placement of V1-2 (negative P-wave in V2), normal conduction and axis, delayed R wave with old Q wave in V2 and aVL, and normal voltages. The emergency physician and final cardiology read noted the inferior ST depression.
Smith: Often “inferior ST depression is attributed to “inferior ischemia” (which implies inferior subendocardial ischemia). But we have shown countless times AND it is proven in the literature that subendocardial ischemia does not localize to any one wall. ST depression in the “inferior” leads represents a reciprocal finding to high lateral (aVL mostly).
Jesse continues: “This ST depression is reciprocal to subtle ST elevation in aVL. And there’s also ST depression in V5-6 which is reciprocal to subtle ST elevation and hyperacute T wave in V1-2 – the precordial swirl pattern.
So a patient with high pre-test likelihood presented with chest pain and new ischemic changes in 8/12 leads. But it did not meet STEMI criteria, so it was signed off as “STEMI negative” and the patient waited to be seen.
I sent the ECG without the prior for comparison, and without any clinical information to Dr. Smith, who immediately replied, “swirl and South African flag” – in other words, both precordial swirl (V1-2 STE/hyperacute T with reciprocal STD V5-6), and South African flag pattern (STE aVL-V2, with reciprocal inferior STD), each of which, independently, are OMI patterns. The latter on its own is typical of diagonal occlusion, but precordial swirl localizes the acute coronary occlusion to the proximal LAD – which is important to interpret what happened next.
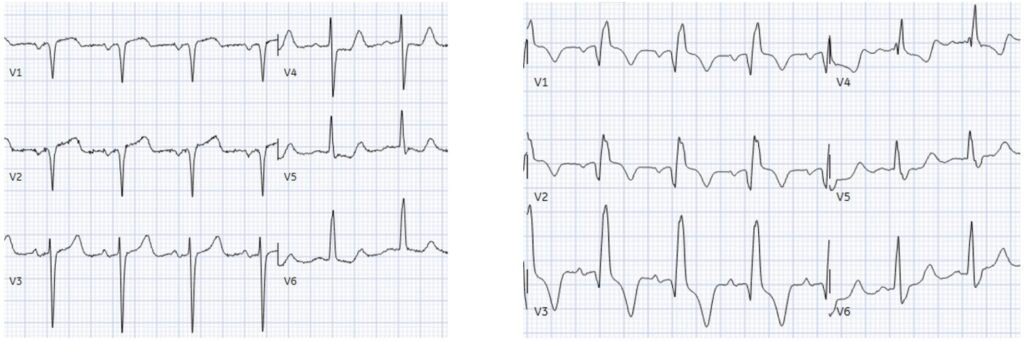
Repeat ECG: acute RBBB
The patient was seen 2 hour later, when the first hs-trop I returned at 400ng/L (normal <26 in males and <16 in females), with ongoing chest pain and a change in the ECG. What do you think?
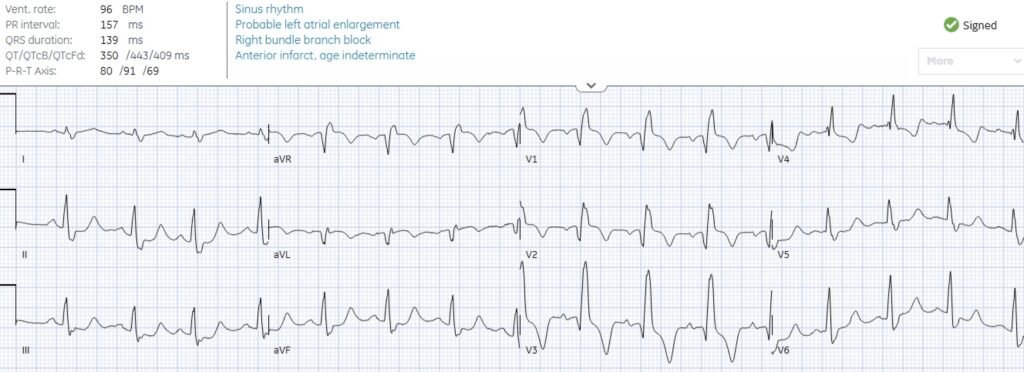
There’s RBBB, Q waves now extend to V3, there’s subtle concordant STE V1-2, followed by T wave inversion. Out of context, this could represent an old RBBB with old LV aneurysm morphology (anterior Q + mild STE + TWI). But the RBBB and the Q wave in V3 were both acute. The TWI including in aVL might indicate reperfusion if the symptoms were fully resolved, but the patient still had ongoing chest pain.
Smith: There is also a deeper and wider Q-wave in aVL.
The Queen of Hearts found both ECGs to be diagnostic of OMI – highlighting the hyperacute T wave on the first, the concordant STE on the second, and the inferior reciprocal change in both:
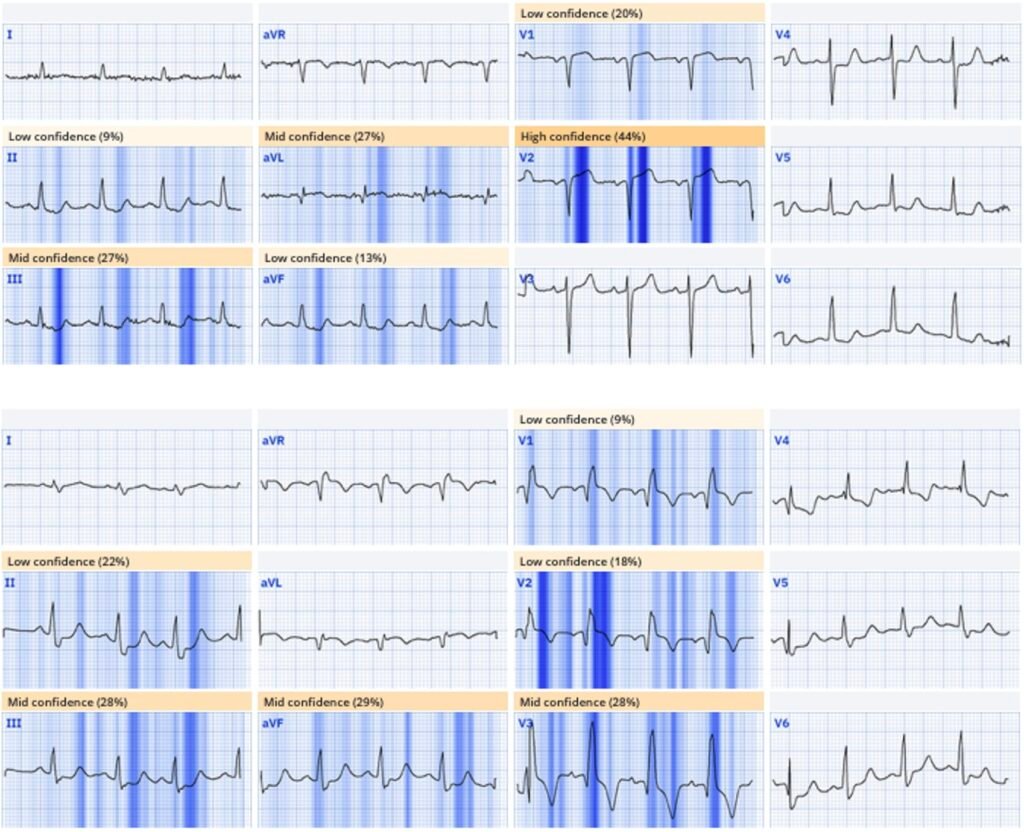
New PMcardio for Individuals App 3.0 now includes the latest Queen of Hearts model and AI explainability (blue heatmaps)! Download now for iOS or Android. https://www.powerfulmedical.com/pmcardio-individuals/ (Drs. Smith and Meyers trained the AI Model and are shareholders in Powerful Medical).
While there is no evidence that “new LBBB” identifies OMI (but the Modified Sgarbossa Criteria does), there is evidence that acute RBBB is a high risk feature of proximal LAD (or left main) occlusion.
ACS with refractory chest pain: CT chest, 15 lead ECG or angiogram?
If the patient had presented with a STEMI positive ECG, the cath lab would have been activated immediately. There were no high risk features suggesting aortic dissection, and STEMI secondary to aortic dissection is extremely rare (0.51% in this study). But because the patient had serial ECGs that were “STEMI negative”, the physician searched for other dangerous causes of chest pain – with a CT to rule out aortic dissection. Repeat ECGs over the new few hours showed ongoing occlusion:
Smith: we proved in this study of complete, total LAD occlusions (published just last week) that hyperacute T-waves do NOT reliably evolve to ST Elevation (0/17 cases in this study!). Meyers HP…Smith SW. Failure of standard contemporary ST-elevation myocardial infarction electrocardiogram criteria to reliably identify acute occlusion of the left anterior descending coronary artery. Eur Heart J Acute Cardiovasc Care. 2025;zuaf037
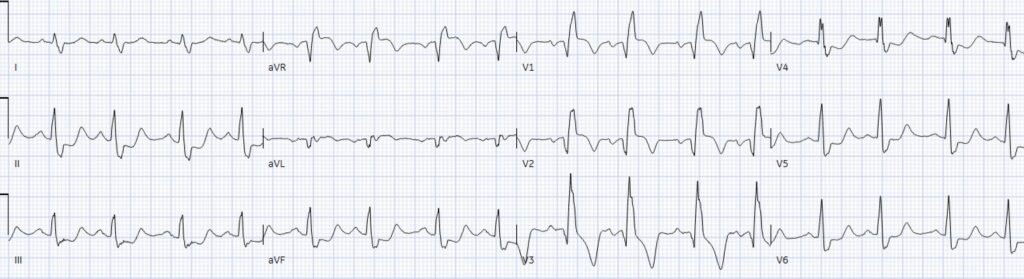
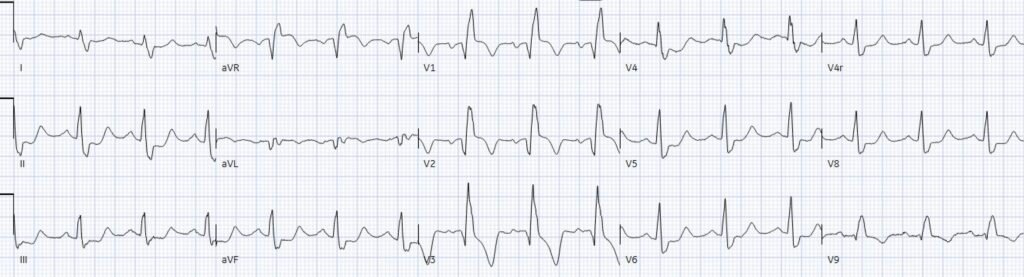
(Note: a 15 lead ECG done for a patient with ‘non-STEMI’ and ongoing pain should qualify for cath lab activation – because this means the patient still has ischemic symptoms and the provider is looking for ST elevation, but ACS with refractory ischemia is an indication for angiography regardless of the ECG). CT chest revealed no dissection and the patient was referred to cardiology.
Non-STEMI or STEMI-negative OMI?
Cardiology noted the patient to have ongoing 2/10 pain 8 hours after arrival, with a new RBBB and troponin rising to 4,000, but admitted the patient as “non-STEMI” because no ECGs ever met STEMI criteria.
Smith: there is no more worthless diagnosis than “Non-STEMI”
Over the next 24 hours the troponin rose to 40,000ng/L, and echo showed EF reduced from 45% to 30% with an akinetic anterior wall. The patient was taken to cath lab to find a 95% proximal LAD occlusion as predicted by the triage ECG. Follow up ECG showed persisting RBBB with anterior Q waves, and resolution of concordant STE:
The patient had a 24 hour delay to reperfusion for a proximal LAD occlusion with acute RBBB, a peak troponin of 40,000ng/L, and an akinetic anterior wall. But the discharge diagnosis remained “non-STEMI”, which perpetuates the current paradigm – rather than STEMI(-)OMI which would flag the case for quality improvement.
Smith: because it was a “Non-STEMI”, it was not a miss. It undergoes no Quality improvement analysis, violates no quality assurance measures, and completely goes beneath the radar. It is considered “appropriate care,” despite worse patient outcomes. This is the “No False Negative Paradox:” If the ECG does not meet STEMI criteria, and there is an occlusion, it is a NonSTEMI and therefore not a false negative STEMI. Can’t be a STEMI because the definition of the disease is based on one aspect (STE) of one test (the ECG). No other pathology would be so defined.
NSTEMI is like saying that appendicitis is a “Low White Count Abdominal Pain.” And if the white count is low, but the appendix perforates, no problem. We didn’t miss it because the WBC count was not high.
Take home
- The first consideration for ischemic STD is reciprocal to subtle STE and hyperacute T waves – including inferior STD reciprocal to subtle high lateral, and V5-6 STD reciprocal to V1-2 (precordial swirl)
- Precordial swirl identifies patients with proximal LAD occlusion at risk of acute RBBB, which is a much higher risk presentation than ‘new LBBB’
- RBBB causes secondary discordant STD/TWI in the anterior leads with RsR’, so looking for concordant STE/hyperacute T in these leads can identify subtle LAD OMI
- If a patient with ACS has refractory ischemia it is highly unlikely to be from aortic dissection if the ECG is diagnostic of OMI, and a 15 lead ECG done for ACS with refractory ischemia means the patient needs an angiogram
- A discharge diagnosis of “non-STEMI” for delayed reperfusion of an acute coronary occlusion is a barrier to quality improvement
======================================
MY Comment, by KEN GRAUER, MD (8/10/2025):
Today’s post by Dr. McLaren reviews the frustrating case of a 75-year old man with known coronary disease — who despite presenting with a 4-hour history of new CP (Chest Pain) — underwent a 24-hour delay until reperfusion even though his initial ECG done a day earlier was already diagnostic of acute OMI.
- Dr. McLaren reviews the series of oversights that unfortunately occurred during this 24-hour period — including most frustratingly the discharge diagnosis of “non-STEMI” for a clear acute OMI that even without access to this patient’s prior baseline tracing — should have been diagnosed immediately given the past medical history of known coronary disease, the present history of severe, persistent new CP — and the initial ED triage ECG that is diagnostic of acute OMI until proven otherwise (without need for Troponin values or even repeat ECG for justification to activate the cath lab).
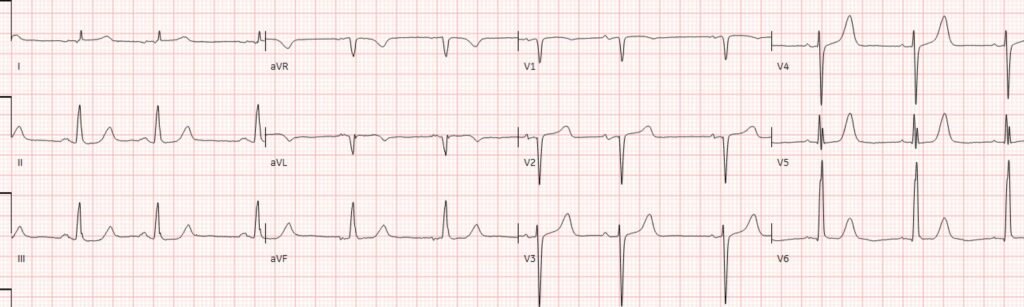
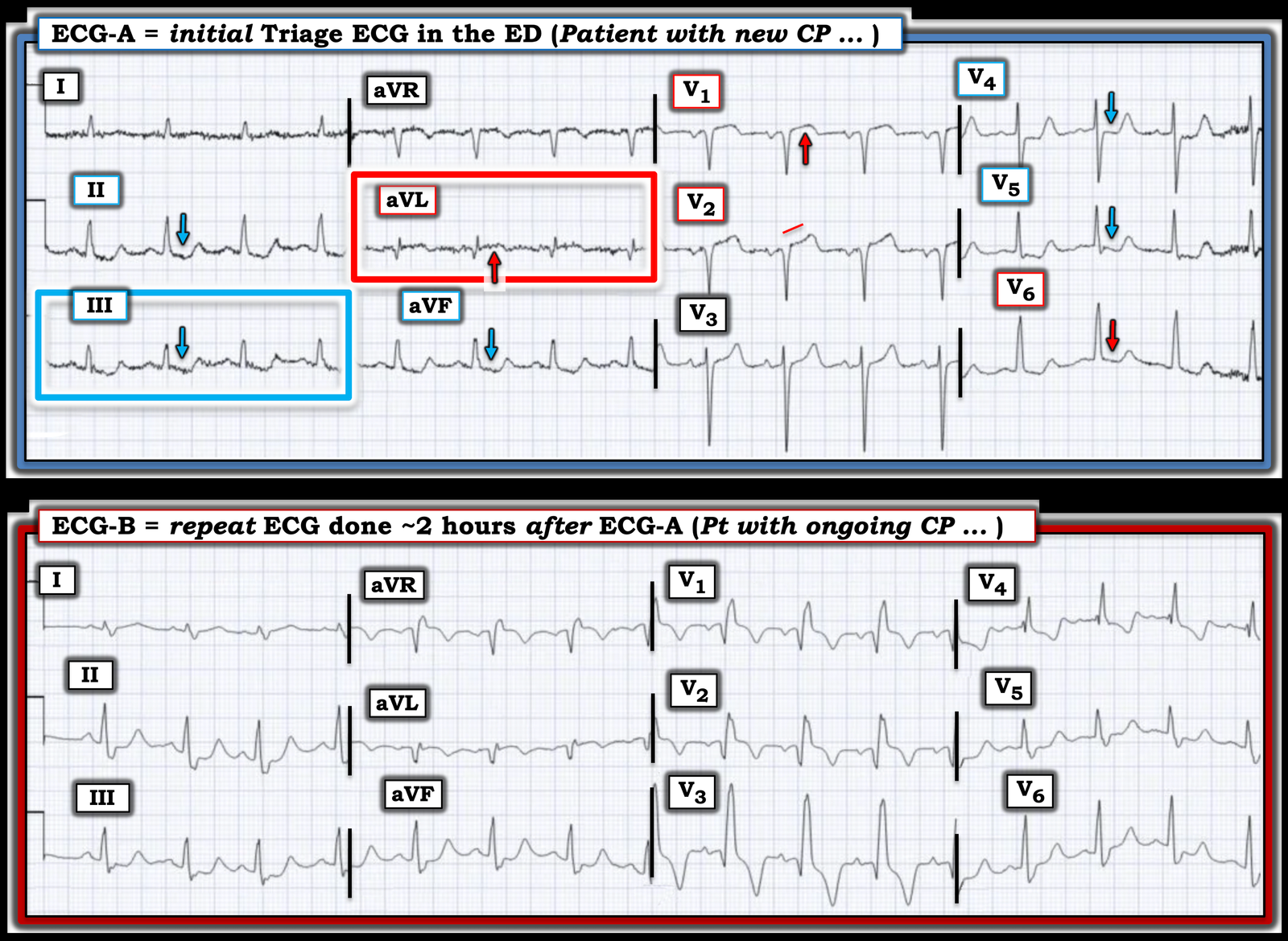
I focus My Comment on some additional points regarding the initial Triage ECG as compared to the repeat ECG obtained ~2 hours later (both of which I’ve reproduced in Figure-1).
= = =
Figure-1: Comparison between today’s initial Triage ECG — with the repeat ECG recorded ~2 hours later.
Today’s Initial Triage ECG:
As per Drs. McLaren and Smith — today’s initial Triage ECG is diagnostic of acute OMI until proven otherwise. While true that the diagnostic ECG findings in ECG-A are somewhat subtle — they have to be recognized!
- Seemingly ignored is the pre-test likelihood (ie, before you even look at this ECG) that this patient is having an acute cardiac event because: i) This older man has known coronary disease with a history of a prior infarction; and ii) This patient now presents to the ED with persistent new CP that has been ongoing for the previous 4 hours.
- Given the above history — any potentially acute ECG abnormalities merit strong consideration for prompt cath (or at the least — more careful lead-by-lead comparison with this patient’s baseline ECG and obtaining a repeat ECG much sooner than the 2 hours that it took to order ECG-B, especially given this patient’s ongoing CP).
- To Emphasize: The findings I cite below were immediately apparent to me before I compared ECG-A with this patient’s baseline ECG.
No less than 9/12 leads in ECG-A show worrisome ST-T wave changes:
- My “eye” was immediately captured by lead aVL (within the RED rectangle in ECG-A). In this patient with ongoing CP — the relatively large Q wave (given tiny size of the QRS in aVL) — in association with ST coving and elevation with terminal T wave inversion has to be assumed acute until proven otherwise.
- Confirmation that the ST-T wave changes in lead aVL are “real” and acute — is forthcoming from the mirror-image oppositve ST-T wave picture in lead III (complete with terminal T wave positivity after the ST depression in this lead).
- Further confirmation is forthcoming from similar ST-T wave changes in the other 2 inferior leads ( = leads II and aVF).
Having established from the limb leads that this patient with persistent new CP is having an ongoing acute cardiac event — I focused my attention on the chest leads.
- There is definite straightening of the ST segment takeoff in lead V2. In addition, given modest depth of the S wave in V2 — there is more than the expected amount of ST elevation in this lead.
- Especially in the context of this ST-T wave abnormality in neighboring lead V2 — the ST segment coving and the amount of J-point ST elevation in lead V1 is clearly abnormal (again by the concept of proportionality — as the S wave in lead V1 is small).
- Finally — the downward arrows in leads V4,V5,V6 highlight ST-T wave straightening and depression in these lateral chest leads.
- As per Dr. McLaren — the combination of ST elevation in leads V1,V2 with ST-T wave depression in lead V6 implicates the pattern of Precordial “Swirl” (See numerous examples of Swirl patterns in the October 15, 2022 post — with a summary in My Comment at the bottom of the page on what to look for in leads V1,2 and V5,V6 to diagnose “Swirl”).
= ==
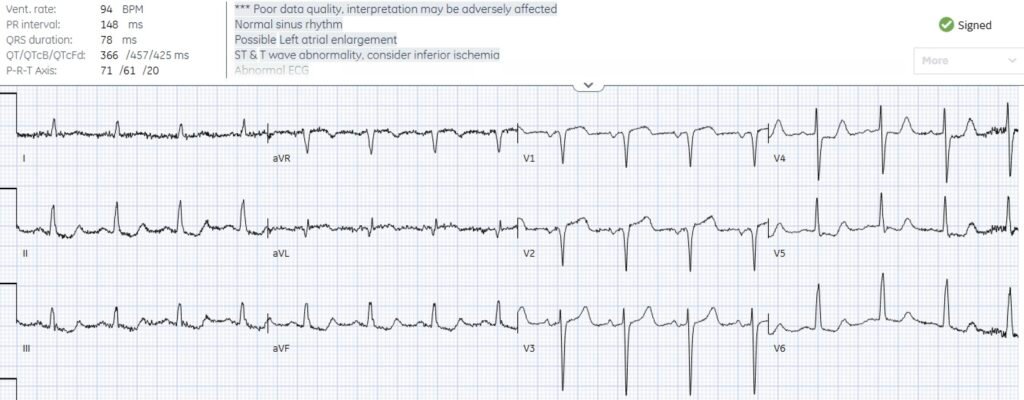
Comparison Between ECG #1 and the Repeat ECG:
As per Dr. McLaren — ECG-B now demonstrates RBBB (Right Bundle Branch Block) that was not present on the initial triage tracing.
- NOTE #1: The 1st thing I checked on seeing the new appearance of RBBB in ECG-B — was the relative heart rate of this repeat ECG compared to the initial triage ECG, since IF the ventricular rate had increased — then this might simply represent a rate-related bundle branch block. But the heart rate is virtually identical in ECG-A and ECG-B. Given this patient’s ongoing CP in association with increased ST-T wave abnormalities — new development of RBBB in a patient with acute proximal LAD OMI (as diagnosed by the “Swirl” pattern described above) is a worrisome sign of infarct progression.
- NOTE #2: Comparison of ECGs-A and -B in Figure-1 provides an excellent illustration of how RBBB represents a terminal delay in conduction. That is, with RBBB — the septum is still depolarized normally (from left-to-right) — which is followed by normal depolarization of the left ventricle — with right ventricular depolarization only now occurring by slow transmission of the electrical impulse from the depolarized LV (ie, because the right bundle branch is blocked — RV depolarization is delayed and occurs slowly by spread over nonspecialized myocardial fibers).
- We see this in ECG-B — because the initial QRS deflections in ECG-B are virtually identical to what they were in ECG-A. It is the terminal delay in RV depolarization with RBBB that produces the wide terminal S waves in lateral leads I and V6 of ECG-B — and the tall R’ deflection in lead V1.
- The relevance of the above 2 bullets — is that while Q waves were present in leads V1,V2 of ECG-A — the Q wave in lead V3 in ECG-B is new!
- That there is ST elevation in leads V1,V2 is now more obvious in ECG-B than it was in ECG-A. This is because the ST-T wave in leads V1,V2 will typically be at least slightly depressed in these leads when there is RBBB. Thus the coved ST segments that we see as elevated in ECG-B have “risen” even more than it may seem because they should have shown some ST depression with development of the RBBB.
Lead-by-lead comparison between ECGs-A and -B in the limb leads shows:
- In lead aVL — a deeper Q wave and greater ST elevation with more terminal T wave inversion than was present in ECG-A.
- In lead I — a hyperacute T wave that was not present in ECG-A (Remember to include proportionality in your assessment of T wave “size” in lead I of ECG-A).
- In the inferior leads — ST segment straightening and deeper ST depression with taller terminal T wave positivity than was seen in ECG-A.
Bottom Line: Any doubt that may have remained about the diagnosis of acute proximal LAD occlusion with worrisome development of acute RBBB — should have been removed once the repeat ECG was recorded.