Written by Jesse McLaren
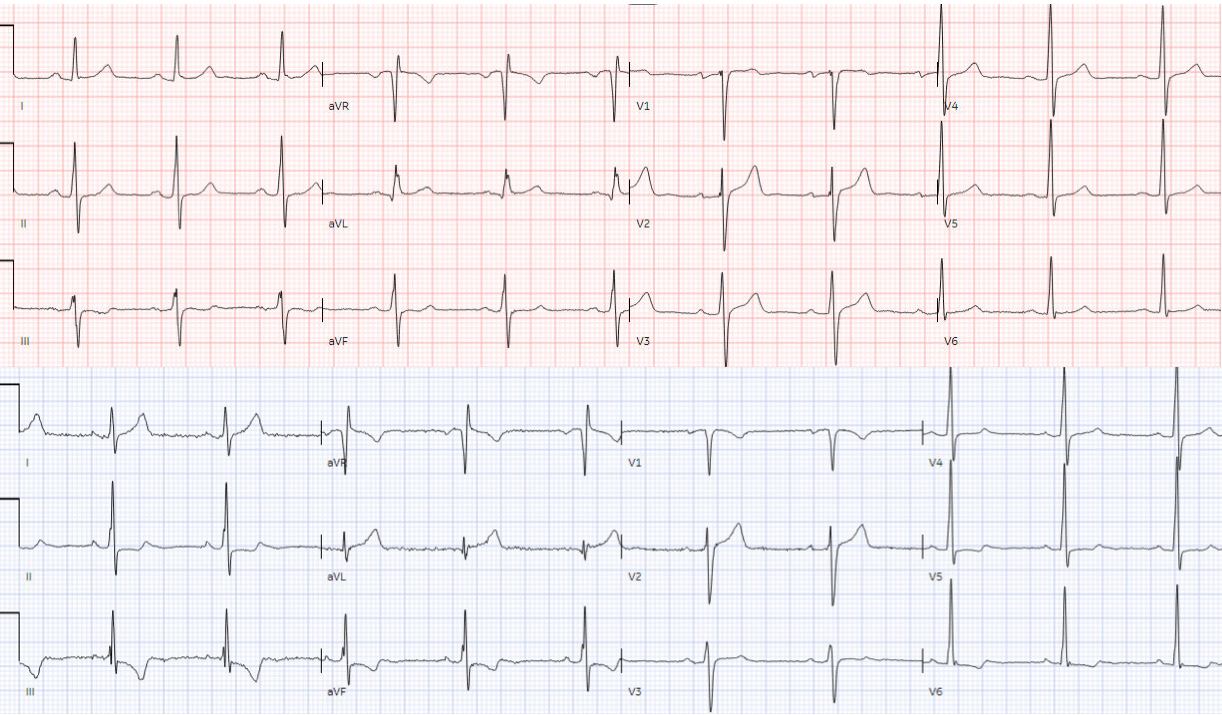
A 45 year old presented with two weeks of recurring
non-exertional chest pain, now constant for an hour. Below is old and then new
ECG (old on top; new below). What do you think?
Both ECGs have normal sinus rhythm, normal conduction and
normal voltages. There’s a change in axis that may interfere with direct
lead-to-lead comparison, but there appear to be larger T waves in I/aVL and new
TWI in III/aVF. But do they represent acute coronary occlusion?
Because of the ECG changes in a patient with chest pain, and
with inferolateral hypokinesis on POCUS, the cath lab was activated. But
coronaries were normal, and serial high sensitivity troponin was undetectable. Formal
echo showed EF 55% with mild inferolateral hypokinesis without any prior for
comparison. Based on ECG changes and echo findings, the patient was diagnosed
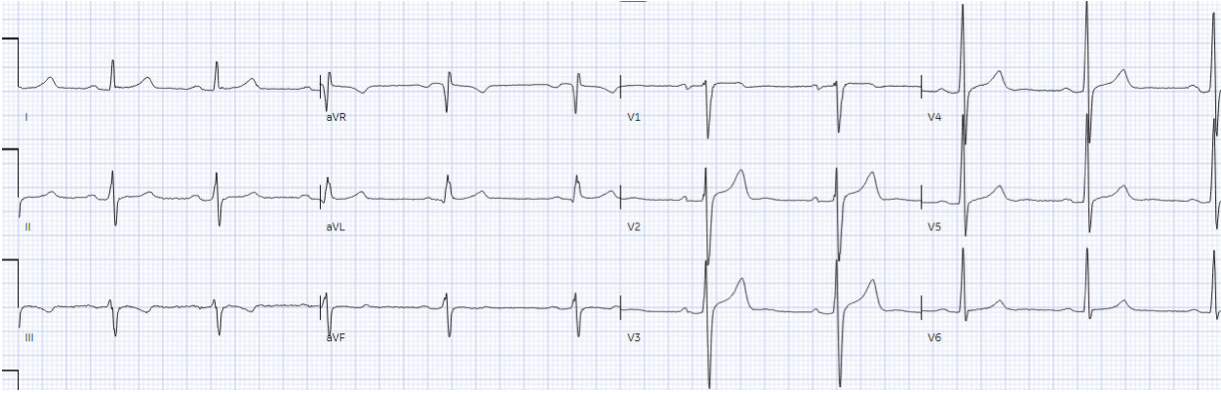
as coronary vasospasm. Below is the discharge ECG, which showed the baseline
ECG without any reperfusion T wave inversion.
The admission and discharge diagnosis both attributed the
ECG changes and echo findings to ischemia. But the echo findings could have
been old (especially with undetectable troponins), and the ECG changes could
have been non-ischemic. This brings up questions around hyperacute T waves and
reciprocal changes, dynamic ECG changes, and the ability to identify
preventable cath lab activations.
OMI vs not OMI: what’s
hyperacute and where’s the reciprocal change?
Leads III/aVL are reciprocal to each other, so any ST/T wave
in one will elicit the opposite in the other. But interpreting which is the
main change, and which is the reciprocal change, can be challenging.
Looking back at the ECG that led to the cath lab activation, the emergency physician interpreted the tall T wave in I/aVL
as hyperacute, and therefore the inferior TWI as the reciprocal change. It was
good to look for hyperacute T waves that are tall relative to the QRS, which
can be small in absolute terms in leads with small QRS like aVL. But these T
waves do not fit the other features of hyperacute T waves: wide based,
symmetric and inflated, with a large area under the curve. Here, the lateral T
waves have a very concave ascent to a narrow peak, with a small area under the
curve. Instead it is the inferior leads that have discordant and asymmetric
TWI, similar to LVH type strain pattern, and this produces reciprocally tall T
(but not hyperacute) T wave in the high lateral leads.
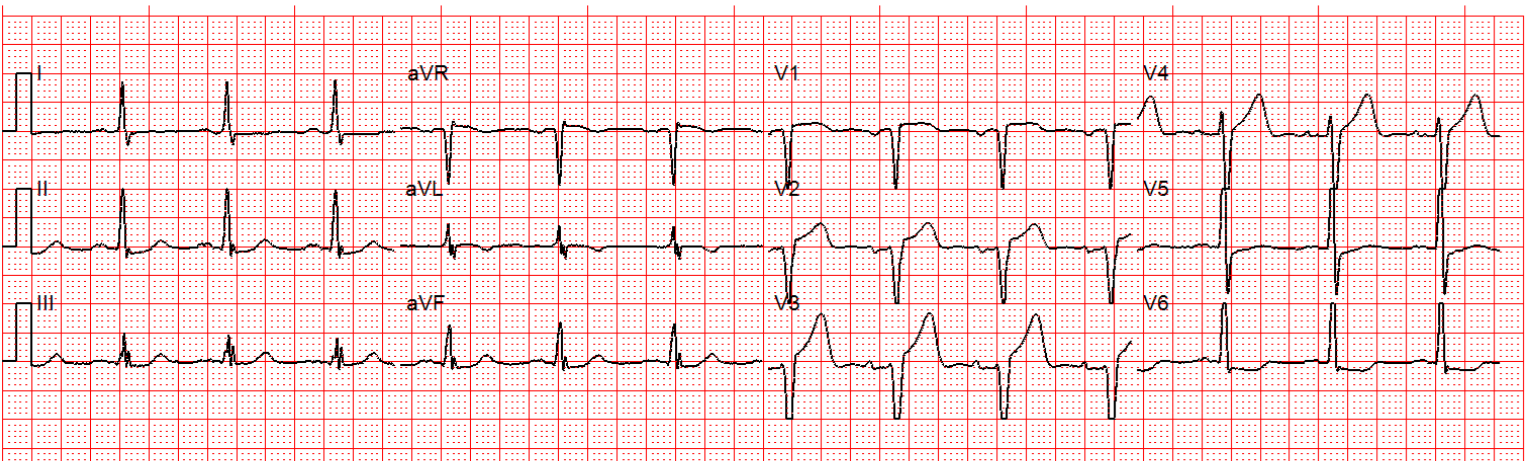
Here’s a comparison with patient with a 100% ramus
occlusion, with lateral hyperacute T waves and inferior reciprocal STD/TWI:
ECG changes: ischemic
vs fluctuating baseline
The other challenge for this case was the change from
previous ECG, which in a patient with chest pain leads us to think of ischemic
change. But looking back in the chart, the patient had prior visits for a
variety of complaints with fluctuating ECG changes similar to the more recent
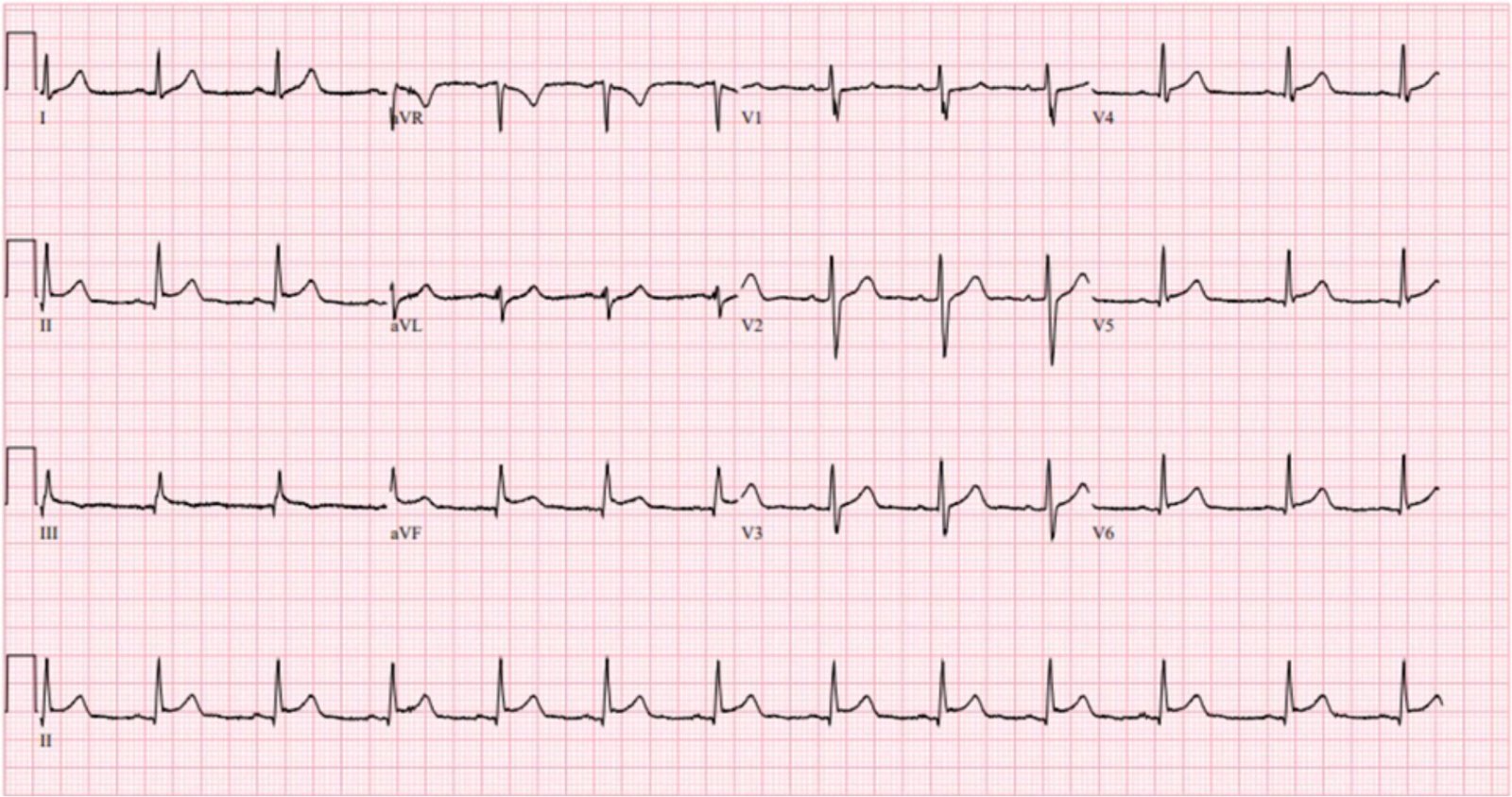
ones, and with troponin levels always undetectable. Here are the ECGs from two
prior visits, the top with chest pain and the bottom with abdominal pain.
Comparison of these two ECGs could also be interpreted as “dynamic”
inferior/lateral changes, when it was fluctuating baseline changes unrelated to
ischemia
Similarly, patients with early repolarization or LVH can
have fluctuating ECG changes over time, which can be mistinterpreted as dynamic
ischemic changes if patients present with chest pain. For this reason, ECGs
need first to be interpreted in isolation, and then applied to the patient.
Could this false
positive cath lab activation been prevented?
The biggest problem with STEMI criteria are false negatives
– because this costs patient’s myocardium, with greater mortality and
morbidity. But the resource cost of false positives is not insignificant, and
the ideal goal would be to identify both false negatives in need of emergent
reperfusion as well as false positives that don’t need cath lab activation. Coronary vasospasm that mimics OMI should not be prevented because this is an angiographic diagnosis of exclusion, but what about vasospasm as a retrospective diagnosis based on an ECG that did not mimic OMI?
I sent both ECGs to Dr. Smith, with the only information
that these were prior vs new ECG. Despite the ECG changes, he immediately
replied, “not OMI.” I also sent the new ECG to the Queen of Hearts App, which
replied with the same answer as its teacher: Not OMI, high confidence.
Patients can still have OMI in the absence of ECG changes –
and ischemic symptoms with regional wall motion abnormalities is a compelling
reason for cath lab activation. But having the reassurance that this ECG in
isolation does not represent acute coronary occlusion might have given pause to
cath lab activation, and to the discharge diagnosis.
Having this expert-trained AI widely available could
dramatically improve the identification of both false negatives and false
positives, saving myocardium and resources at the same time.
Take home
1.
when there are changes in reciprocal leads,
consider which is the main changes and which is the reciprocal change:
hyperacute T waves can produce reciprocal TWI, and TWI can produce reciprocally
tall T waves
2.
hyperacute T waves are tall relative to their
QRS, as well as bulky/inflated with a large area under the curve
3.
baseline ECGs may fluctuate over time, and not necessarily
represent dynamic ischemia
4.
the QoH can help identify both false negative
and false positives
The Queen of Hearts PM Cardio App is now available in the European Union (CE approved) the App Store and on Google Play.
For Americans, you need to wait for the FDA. But in the meantime:
YOU HAVE THE OPPORTUNITY TO GET EARLY ACCESS TO THE PM Cardio AI BOT!! (THE PM CARDIO OMI AI APP)
If you want this bot to help you make the early diagnosis of OMI and save your patient and his/her myocardium, you can sign up to get an early beta version of the bot here. It is not yet available, but this is your way to get on the list.
https://share-eu1.hsforms.com/18cAH0ZK0RoiVG3RjC5dYdwfyfsg
===================================
MY Comment, by KEN GRAUER, MD (11/27/2023):
===================================
Superb commentary by Dr. McLaren — that addresses a number of truly challenging aspects regarding ECG assessment of patients who present with new chest pain. I’d add the following to Dr. McLaren’s thoughtful and insightful commentary.
- It is highly likely that the 45yo in today’s case will have one or more recurrences in the future. Given the accumulating “data set” of serial ECGs from this admission — and, from prior visits — apparently always with undetectable Troponin — and, with cardiac cath on this admission not showing any significant coronary disease — future disposition decision-making is simplified. As long as Troponin is negative and ECGs continue to show the similar pattern of ST-T wave variation seen on this admission — future cardiac catheterization should be avoidable.
- Dr. McLaren’s description of serial ECG assessment of the first 2 tracings in today’s case ( = the “old” then “new” ECGs) — is a model for clinicians to emulate. Lead-by-lead comparison is tremendously facilitated by Dr. McLaren’s technique of putting the 2 ECGs to be compared side-by-side. All too often I observe clinicians look entirely at 1 tracing, not seeing anything — and then looking entirely at the 2nd tracing without ever putting both tracings next to each other. This faulty technique renders it very easy to miss potential “dynamic” ST-T wave changes between these 2 tracings — that if interpreted separately, would not raise suspicion.
- Dr. McLaren astutely points out the difference in frontal plane axis between the first 2 tracings — which must be taken into account when assessing whether ST-T wave appearance in serial tracings is truly changed vs simply different due to a change in frontal plane axis. I completely agree with Dr. McLaren that the changes between the “old” and “new” ECG seem more than what might be explained solely by a shift in frontal plane axis.
- There are some subtle ST-T wave changes also in the chest leads. While I suspected the small changes in QRST morphology in leads V1,V2,V3 in the first 2 tracings were unlikely to be significant (if real at all — given slight difference in R wave progression) — I thought there clearly was change from the upright T waves in leads V4,V5,V6 in the top tracing — to the ST segment flattening, with slight ST downsloping in lead V6 of the bottom tracing that looks typical for LV “strain” (despite lack of voltage criteria for LVH).
- I would want to know formal Echo specifics as to whether this patient has LVH (as per Dr. McLaren — patients with LVH are more prone to fluctuating ECG changes over time, that are more likely to be misinterpreted as dynamic ischemic changes). As a side note — I occasionally do see LV “strain” in patients with Echo-confirmed LVH, who do not satisfy LVH voltage criteria.
- Finally — I think it important to point out that while the serial ECGs in today’s case were not indicative of acute OMI — neither ECG is “normal”. The essential “immediate” decision to be made in “zero time” in the ED, is whether or not prompt cath and reperfusion is needed. That said — I like to look for an explanation as to WHY a given ECG looks the way it does in patients who present for chest pain. Today’s patient clearly has variable ST-T wave appearance over time — and, despite normal coronary arteries and a respectable EF ~55% on formal Echo — there is at least mild inferolateral hypokinesis. It may be in this case that no satisfying explanation can be found beyond determination of “not being ischemic” — but additional information may be insightful.
- BOTTOM Line: Our confidence is enhanced from today’s case that future visits by this patient with a similar presenting history, negative troponin values and similar variations on serial tracings — will not indicate OMI (and should not need cardiac cath). That said — IF I was charged with evaluating a 45yo with a 2-week history of non-exertional chest pain, now constant — with inferolateral hypokinesis on POCUS and similar initial and prior tracings as shown at the beginning of today’s case without the benefit of previous visits with prior ECGs to compare — I would have trouble being 100% certain that the ECG appearance with serial ST-T wave changes were not ischemic. While “fake” rather than OMI may seem the more likely interpretation (as per Drs. McLaren and Smith and the QOH no OMI interpretation) — I would probably favor a cath to be certain (with the knowledge that performing an anatomy-defining cardiac catheterization should facilitate and expedite future decision-making and avoidance of repeat cath on future visits).
GREAT case by Dr. McLaren!