Written by Jesse McLaren, with comments by Grauer
A 60 year old had two hours of exertional chest pain, nausea
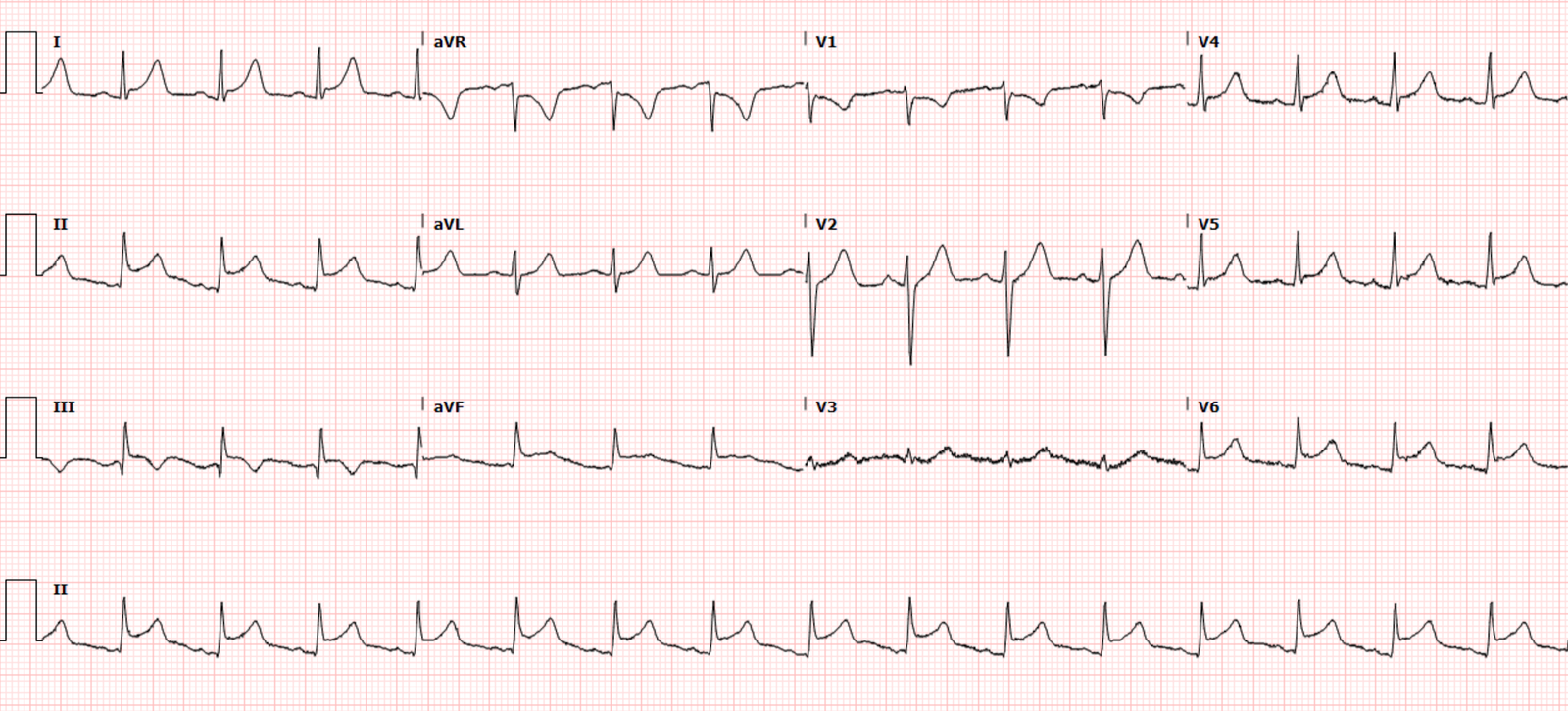
and diaphoresis, and called EMS. Below is the first ECG on arrival to hospital,
and vitals were normal except for HR of 100. What do you think?
There’s borderline sinus tach, incomplete RBBB, normal axis,
normal R wave progression but small Qs in V2-3 (which should never be present),
and normal voltages. There’s ST elevation and hyperacute T waves in V2-4, and
mild ST elevation inferiorly. By STEMI criteria alone this is borderline (which
is why it was not labeled STEMI in the final cardiology interpretation), but
the additional signs of OMI (pathological Qs and hyperacute Ts) make this
diagnostic of LAD occlusion—beyond the first diagonal, so there is no injury to
the lateral wall and no inferior reciprocal change.
According to the paramedic report, their ECG (which was not
scanned into the chart) showed “marked anterior ST elevation” so the patient
was taken directly to the cath lab where the ECG above was recorded, when the
patient reported that their pain was improving. On angiogram 25 minutes after the
ECG above there was a hazy and eccentric mid LAD lesion but it was only 30%
stenosed, so no PCI was done. There was also a 85% stenosis of an obtuse
marginal branch, which was not thought to be acute or contributing to the
patient’s presentation.
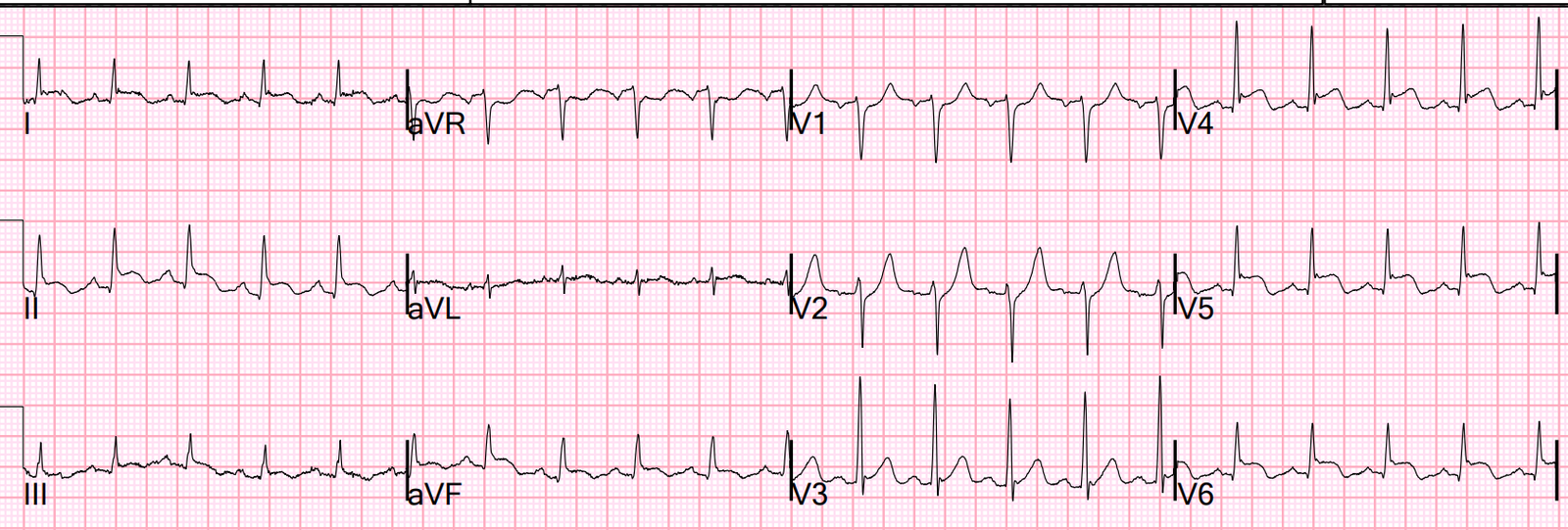
Here is the post-angiogram ECG, showing further development
of Q waves V2-3 and some deflating of hyperacute T waves:
First hs-troponin I was 2400ng/L (normal <26 in males and
<16 in females) and repeat 5 hours later was > 50,000 (the upper level of
recording), with apical akinesis. So this was a very large MI with
non-obstructive coronary arteries (MINOCA). What was the cause?
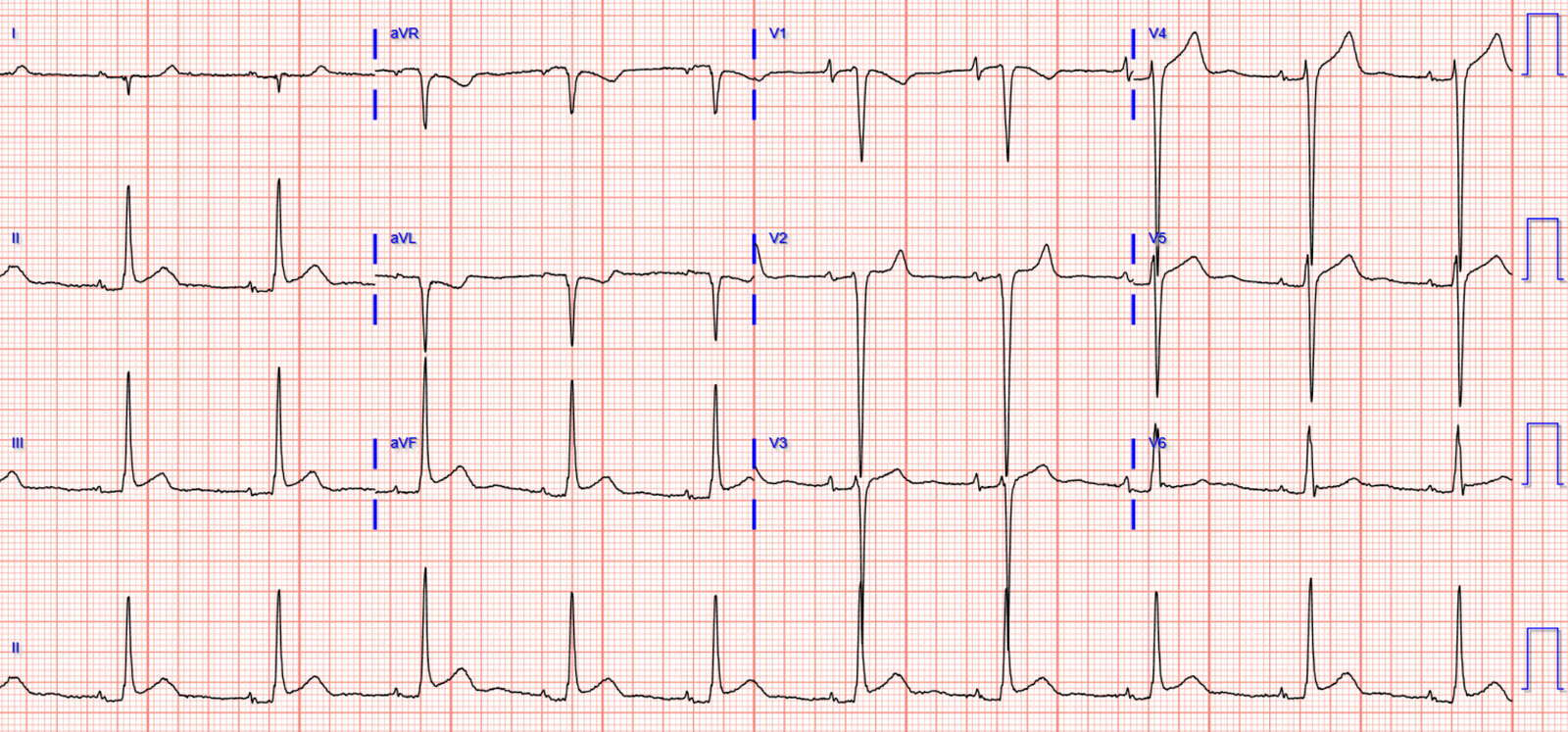
8 hours after the angiogram the patient developed chest
pain again. Repeat ECG:
There is now total loss of anterior R waves, with full QS
waves in V2-3 from evolving anterior MI. But there is also a hyperacute T wave in
V3 (T/QRS>0.36) indicating acute occlusion [1], with terminal T wave inversion
V2-4 suggesting some reperfusion.
The patient was taken back to the cath lab, but by the time
of the angiogram the LAD lesion was again non-occlusive—though this time 60%.
The troponin was still in the >50,000 range and only 8 hours after the first
event so could not be relied on to identify a second event. But based on
symptoms, ECG changes and progression of stenosis the patient received a stent
to their mid LAD.
Here is the next day ECG showing reperfusion T wave
inversion:
Below is the discharge ECG three days later, showing
reappearance of anterior R waves:
The patient was discharged with a diagnosis of STEMI even though their ECG was borderline for STEMI criteria and despite
two angiograms showing TIMI 3 flow. The patient had no further MIs on follow up.
MINOCA
The term “STEMI”
is equated with acute coronary occlusion. But 25% of “Non-STEMI” have near or
total occlusion with insufficient collateral circulation, with absent or
reduced flow (TIMI 0-2) on angiogram[1]. And 15% of “STEMI” have open arteries
with normal TIMI 3 flow [2,3,4]
Then there are MIs
with less than 50% stenosis at the time of angiography, who fall under the term
MINOCA. As a recent article summarized, “Approximately 6–10% of patients
with features of acute myocardial infarction do not show evidence of an obstructed
vessel on angiography. In recent years, these patients have been grouped as
presenting with Myocardial Infarction with Non-Obstructive Coronary Arteries
(MINOCA). The diagnosis of MINOCA is based on three main criteria: (1) clinical
evidence of MI such as symptomatic chest pain, and ECG changes; (2) coronary
angiography demonstrating no / ≤50% epicardial coronary stenosis and; (3) no overt
cause of AMI presentation…There is a wide plethora of causes of MINOCA, with
the common ones stipulated as coronary plaque disruption (encompassing plaque
rupture, erosion and calcified nodules), coronary dissection, coronary spasm,
microvascular dysfunction, coronary embolism, thrombophilia states.”[5]
In other words, the first differential for MINOCA is Occlusion
MI—which has lysed but is at risk for re-occlusion. This includes small lesions
like this case, or no obvious intraluminal stenosis in cases with extraluminal
disease like this
case. Or as Dr. Smith explained in this
case, “the clinical presentation
of sudden chest pain, typical ECG findings of occlusion (hyperacute T-waves in
this case), ECG findings in a coronary distribution, rise and fall of troponin
with peak in the typical range for STEMI/OMI, and new wall motion abnormality
in the area indicated by the ECG, must be considered to be due to coronary
thrombosis. The degree of stenosis is not a great predictor of
thrombosis, and culprits may not be visible. Even if there is a tight
stenosis, it is not proof of culprit, as many individuals have tight fixed
stenoses at baseline. There may be a chronic tight stenosis and a
non-obstructed lesion that thrombosed.”
Similarly, in the case presented above the ECG revealed mid
LAD OMI which reperfused by the first angiogram, then the ECG revealed
recurrent OMI that reperfused by the second angiogram, and then the ECG revealed
the expected reperfusion changes after the angiogram. The troponin and echo
findings revealed the aftermath of a large infarct, with apical akinesis corresponding to the ECG changes. But the timing
of the angiograms did not capture the moments of LAD occlusion before
spontaneous reperfusion, and revealed an incidental OM stenosis.
In a study of more than 9000 MINOCA patients, 6.3% had a new
MI on follow up. Of those who had a repeat angiogram approximately half had
recurring MINOCA but the other half had progression of coronary artery
disease.[6] This only includes those discharged with a diagnosis of MINOCA who
had a new MI on follow up, not patients like ours above who had an initial
diagnosis of MINOCA that was changed to STEMI because of a recurrent MI during
the same admission.
Take away
1.
LAD
occlusion often doesn’t meet STEMI criteria or have inferior reciprocal change
(if distal to the first diagonal). But early Q waves (which can be acute and
reversible) and hyperacute T waves can help make the diagnosis
2.
When
there are anterior QS waves with persisting ST elevation, hyperacute T waves
(T/QRS>0.36) can help identify acute occlusion and help differentiate from LV aneurysm morphology
3.
OMI
is not an angiographic finding but rather a clinical diagnosis that
combines symptoms, ECG evolution of occlusion/reperfusion, echo, troponin and
angiographic findings – and the delayed timing of angiography can miss OMI diagnosed at the bedside
4.
Instead
of classifying MIs as “STEMI” (15% of which have open arteries) and “NSTEMI”
(25% of which have occluded arteries), we should classify as OMI (occlusion or
near occlusion with inadequate collateral circulation, with TIMI 0-2 flow or
peak troponin >10,000ng/L) or NOMI
5.
The
first differential for MINOCA is reperfused OMI at risk for reocclusion
References
1.
Smith.
T/QRS ratio best distinguishes ventricular aneurysm from anterior myocardial
infarction. Am J Emerg Med 2005
2.
De
Luca et al. Implications of pre-procedural TIMI flow in patients with non
ST-segment elevation acute coronary syndromes undergoing percutaneous coronary
revascularization: insights from the ACUITY trial. Int J Cardiol 2013
3.
De
Luca et al. Preprocedural TIMI flow and mortality in patients with acute
myocardial infarction treated with primary angioplasty. JACC 2004
4.
Hashimoto
et al. Pre-procedural thrombolysis in myocardial infarction flow in patients
with ST-segment elevation myocardial infarction: a J-MINUET substudy. Int Heart
J 2018
5.
Pravda
et al. Temporal trends in the pre-procedural TIMI flow grade among patients
with ST-segment elevation myocardial infaraction – from the ACSIS registry. IJC
Heart & Vasc 2021
6.
Editorial
“MINOCA” the Pandora’s box. Int J Cardiol 2022
7.
Nordenskjold
et al. Reinfarction in patients with myocardial infarction with nonobstructive
coronary arteries (MINOCA): coronary findings and prognosis
===================================
Comment by KEN GRAUER, MD (6/5/2022):
===================================
Masterful discussion by Dr. McLaren of an unappreciated entity that has become increasingly important to recognize. As emphasized by Dr. McLaren — MINOCA ( = MI with Non-Obstructive Coronary Arteries) occurs in 5-15% of patients presenting with acute ST elevation or non-ST elevation MIs (Sykes et al — Intervent. Cardiolog Rev 16:e10, 2021).
On a philosophical note — the “beauty” of Dr. McLaren’s discussion derives from the sum of his “Take-Aways” — which highlight the “art” of clinical ECG interpretation:
- Even when cardiac cath fails to confirm acute coronary occlusion — deductive ECG interpretation over the course of serial tracings — in conjunction with the clinical history, Echo results, serial troponins — and — the timing of chest pain episodes in relation to the timing of cardiac catheterization(s) — can provide an accurate presumptive answer to the sequence of events in a given clinical case.
- As per Dr. McLaren — the initial ECG in today’s case was diagnostic of acute OMI despite not satisfying millimeter stemi criteria. So it was surprising when cardiac cath done just 25 minutes after this initial ECG revealed nothing more than a 30% mid-LAD lesion with incidental disease in another distribution.
- Regardless of this “negative” cath result — marked troponin elevation with associated apical akinesis left no doubt about the extensive acute infarction.
- In Conclusion: Correlation of the recurrence of this patient’s chest pain with evolving ECG changes (loss of anterior R waves, waxing and waning of Q waves — and dynamic change in shape and relative amount of ST segment deviation) — was enough to justify the determination of an “acute STEMI” despite no more than borderline ECG criteria for this diagnosis and non-obstructive coronary disease on 2 catheterizations.
- Clinically — Stenting of the non-obstructive 60% mid-LAD lesion effectively prevented further infarction. Dependence on millimeter-based stemi criteria without integration of all clinical data (as described by Dr. McLaren) — may have resulted in a far worse clinical outcome.