Written by Jesse McLaren
A previously healthy 50 year-old presented
with 24 hours of intermittent exertional chest pain, radiating to the arms and
associated with shortness of breath. It was ongoing on arrival in the emergency
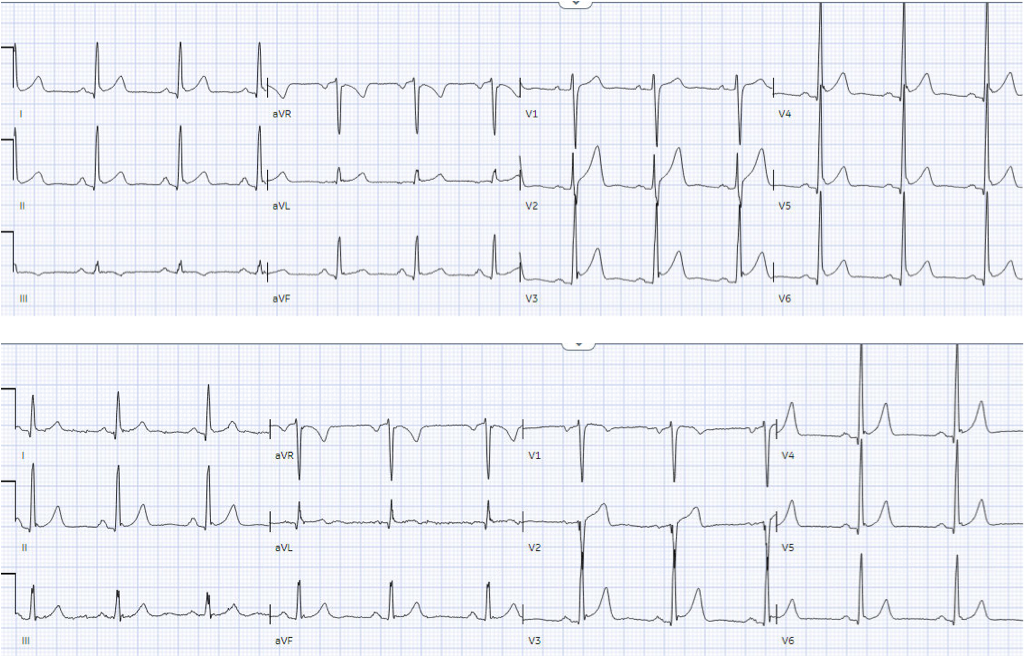
department. Below is the old ECG (on top) and then new ECG (on bottom). What do
you think?
There is normal sinus rhythm, normal
conduction, normal axis, and tall precordial voltages with J waves from early
repolarization. The old ECG has proportional ST elevation and T waves. But the
new ECG has new Q waves in aVL and V2 (the distribution of the first diagonal
artery) – and in the next context of Q waves, the T wave in V2 is upright and
relative large. In a previously healthy patient with new and ongoing chest
pain, this is concerning for acute occlusion of the first diagonal artery.
Smith: Normal ST Elevation in V2-V4 never has an associated Q-wave! So this STE cannot be considered normal even though there was STE on the previous ECG.
But because there was no new ST elevation,
the ECG was signed off as “STEMI negative” and the patient waited to be seen.
The emergency physician was called to see the patient 90 minutes later after
the troponin I returned at 1100 ng/L. This confirms these Q waves are caused by
an acute infarct. But is the artery still occluded?
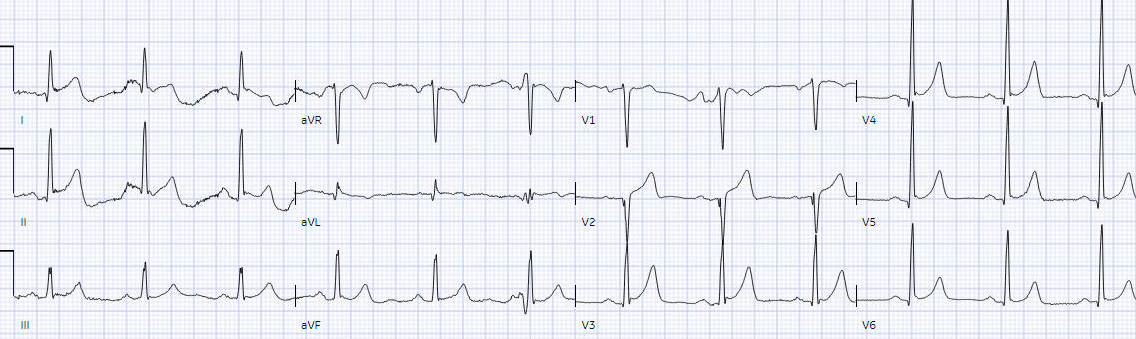
The emergency physician noted the patient
had improved but ongoing chest pain, and had the following repeat ECG:
Ongoing large T wave in V2 with ongoing
symptoms = still an occluded artery. But the ECG still doesn’t meet STEMI
criteria. It was therefore interpreted as “no STEMI” and the patient was
treated with dual anti-platelets and referred to cardiology as “NSTEMI.”
Four hours after arrival cardiology saw the
patient, with a repeat troponin that rose to 1900 ng/L. The patient was now
painfree after anti-platelets and first two ECGs were interpreted as “no
ischemic changes”. So they were admitted as “NSTEMI” with a plan to repeat
troponin levels every 6 hours and perform angiography in 24-48 hours.
Was there spontaneous reperfusion? This
would require resolution of chest pain and reperfusion on ECG, but would still
be at risk for reocclusion. But no repeat ECG was done after symptom
resolution. Eight hours after arrival the patient complained of recurring pain,
with troponin now at 5,200 ng/L, and was treated with a nitro patch. This is
concerning for reocclusion, but there was no repeat ECG and no change in the
plan to wait for angiography.
The next ECG was done 21 hours after
arrival, after troponin peaked at 7400ng/L and then declined to 7000. This ECG
was recorded when the patient was painfree:
Now we see reperfusion T wave inversion in
aVL and V2, consistent with spontaneous reperfusion in a patient who was
painfree. But the biphasic T waves in V2 are not “Wellens T waves” because they
are preceded by acute Q waves and already a large infarct by troponin. This
again confirms there is a critical lesion in the first diagonal, which is
either transiently open or is still occluded but being perfused through
collateral circulation.
But there were no more ECGs for the next 8
hours before angiogram, and no further troponin levels for the remainder of the
patient’s admission.
Angiogram found a totally occluded first
diagonal artery, consistent with all the ECGs, which reperfused after stenting.
Smith: the ECG and troponin suggest reperfusion but the artery remains fully occluded. This is usually a result of restoration of flow from collaterals. This still image of the angiogram is not adequate to comment on collateral flow.
Next day the patient was discharged with a
diagnosis of “NSTEMI” despite a totally occluded artery, and unknown peak
troponin. Fortunately the periodic spontaneous reperfusion before the angiogram
prevented the infarct from being much larger, and allowed the R wave to
reconstitute on the discharge ECG (a final reminder that the initial Q wave was
acute).
In summary, this patient’s occlusion was
missed by
1.
the
computer interpretation
2.
the
emergency physician who signed off the ECG
3.
the
treating emergency physician
4.
the
cardiologist
5.
and worst
of all, the discharge diagnosis that did not change despite an angiogram
showing a 100% occlusion. This is not unusual but standard of care under the
STEMI paradigm, where discharge diagnoses change to highlight false positive
STEMI but not false negative STEMI
I sent the baseline and presenting ECG to
the Queen of Hearts, without benefit of any clinical information (other than
pre-test likelihood on which it is trained), and without the benefit of
comparing with the old ECGs:
So in isolation and without context the ECG is not diagnostic of OMI. But with comparison to prior (which could be incorporated into future versions of the Queen of Hearts) and applied to clinical context it is diagnostic.
Take home
1. ECGs are a snapshot in time, should be compared to prior, and should be repeated if symptoms are ongoing (to look for occlusion) or resolved (to look for reperfusion)
2.
STEMI
criteria is bad at differentiating between normal variant and acute coronary
occlusion or reperfusion, and initial troponin levels don’t differentiate between occlusive and non-occlusive MI
3.
the presence
of J waves from early repolarization doesn’t rule out an acute coronary occlusion
4.
Q waves can
be acute and can resolve with reperfusion
5.
T waves
require context: the same size T wave can be proportional to a normal QRS, or
disproportionate relative to small QRS or Q wave
6.
Spontaneous
reperfusion is still a high risk state, and can have a totally occluded artery on angiogram
7.
The
diagnosis of NSTEMI misses OMI in real time, and does not change
retrospectively to highlight false negative STEMI
8.
QOH can
make expert OMI interpretation widely available
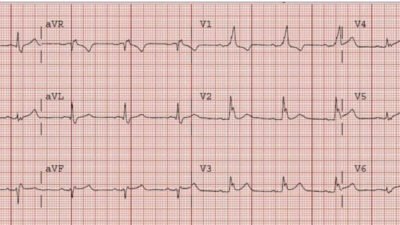
Here is another great example of Q-wave evolution that is missed:
Acute MI from LAD occlusion, or early repolarization?
This is clearly OMI but was not recognized
Then 70 minutes later:
New Q-waves, still not recognized.
These are easy for the Queen:
The Queen of Hearts PM Cardio App is now available in the European Union (CE approved) the App Store and on Google Play.
For Americans, you need to wait for the FDA. But in the meantime:
YOU HAVE THE OPPORTUNITY TO GET EARLY ACCESS TO THE PM Cardio AI BOT!! (THE PM CARDIO OMI AI APP)
If you want this bot to help you make the early diagnosis of OMI and save your patient and his/her myocardium, you can sign up to get an early beta version of the bot here. It is not yet available, but this is your way to get on the list.
https://share-eu1.hsforms.com/18cAH0ZK0RoiVG3RjC5dYdwfyfsg
References
1.
McLaren et
al, including Meyers/Smith. Missing occlusions: quality gaps for ED patients
with occlusion MI. Am J Emerg Med 2023
2.
Herman,
Meyers, Smith et al. International evaluation of an artificial
intelligence-powered electrocardiogram model detecting acute coronary occlusion
myocardial infarction. Eur Heart J Dig Health 2023
![]()
===================================
MY Comment, by KEN GRAUER, MD (12/30/2023):
===================================
Superbly documented case by Dr. McLaren — in which the unfortunate details of opportunities missed are sequentially recounted.
- Given the answers provided by cardiac catheterization — I thought review of the first 3 tracings in today’s case especially insightful. For clarity and to facilitate comparison in Figure-1 — I’ve put these initial 3 tracings together.
 |
| Figure-1: I’ve put the first 3 ECGs in today’s case together. |
MY Thoughts on the first 3 ECGs in Today’s Case:
Based on the first 2 ECGs in today’s case ( = the initial ED tracing — and comparison with an “old” ECG on this patient) — Dr. McLaren correctly predicted acute OMI of the 1st Diagonal Branch of the LAD. I fully acknowledge that — I did not predict the “culprit” vessel until much later in today’s course.
- We have previously shown many cases involving acute OMI of the 1st or 2nd Diagonal Branch of the LAD. These case are most readily recognizable by the South African Flag distribution of ST-T wave deviation (ie, ST elevation in leads I, aVL and V2 — with reciprocal ST depression in lead III — and no ST elevation in chest leads other than V2 — as detailed in the May 11, 2022 post by Dr. McLaren and myself).
- KEY Point: This typical pattern of D1 or D2 OMI is masked in today’s tracing because of a “baseline” ECG ( = ECG #2 in Figure-1) that shows: i) Marked QRS increased amplitude in the lateral chest leads (R waves in leads V4,V5,V6 all exceeding 20 mm); ii) Early transition (Transition already by lead V2 in ECG #2, with R wave height exceeding S wave depth); — and, iii) A baseline repolarization variant, with 1-2 mm of J-point ST elevation in multiple leads. Given these findings on the “old” ECG — it would seem difficult to rapidly evolve an acute tracing with the poor R wave and ST elevation isolated to lead V2 that one would expect with acute D1 or D2 OMI.
- The “baseline” tracing in today’s case already showed what I thought was a disproportionately large T wave by lead V2 (this T wave being taller than the R wave in lead V2 of ECG #2 — and much taller than the S wave in this lead is deep). That said — in the setting of J-point repolarization ST elevation and markedly increased R wave amplitudes beginning in neighboring lead V3 — I did not think anything in the “old” ECG looked acute. But I also did not perceive the T wave in lead V2 of ECG #1 to necessarily be abnormal in view of T wave appearance in lead V2 of ECG #2.
- I perceived the fragmented QS in lead V2 of ECG #1 to clearly be of potential concern. That said, given isolation of this finding to lead V2 — I was not convinced this QS was real (and not the result of lead placement) — until I saw the identical-looking fragmented QS in lead V2 of ECG #3.
The Acute Findings I Focused on in ECG #1:
What convinced me of an acute ongoing cardiac event was comparison of the limb leads between the initial ECG in today’s case and the “old“ ECG:
- For me — the KEY lead in ECG #1 was lead aVL. Despite no significant change in frontal plane axis between ECGs #1 and #2 — there was no denying reduced T wave amplitude with beginning T wave inversion in ECG #1 compared to the “old” ECG (BLUE arrow in lead aVL of ECG #1).
- In view of this definite new change in lead aVL — there was no denying increased T wave amplitude in each of the inferior leads in ECG #1 compared to T wave appearance in the “old” ECG.
- My Impression: Although I was uncertain about a “culprit” artery — in this patient with new and persistent CP — the indication for prompt cath was clear.
The 3rd ECG:
Movement artifact rendered interpretation of ECG #3 difficult in the limb leads.
- KEY findings for me in ECG #3 included; i) The identical fragmented QS in lead V2 (that I thought confirmed the loss in R wave from lead V1 compared to the “old” ECG was a real and important finding); — and, ii) What I felt was a real and significant increase in T wave amplitude in lead V2 of ECG #3 compared to T wave amplitude in ECG #2 — consistent (as per Dr. McLaren) with 100% occlusion of D1, as found on cardiac catheterization.