Submitted by Matthew McArthur, recent emergency physician graduate and regular reader of Dr. Smith’s ECG Blog, ECG Cases, and ECGweekly workout.
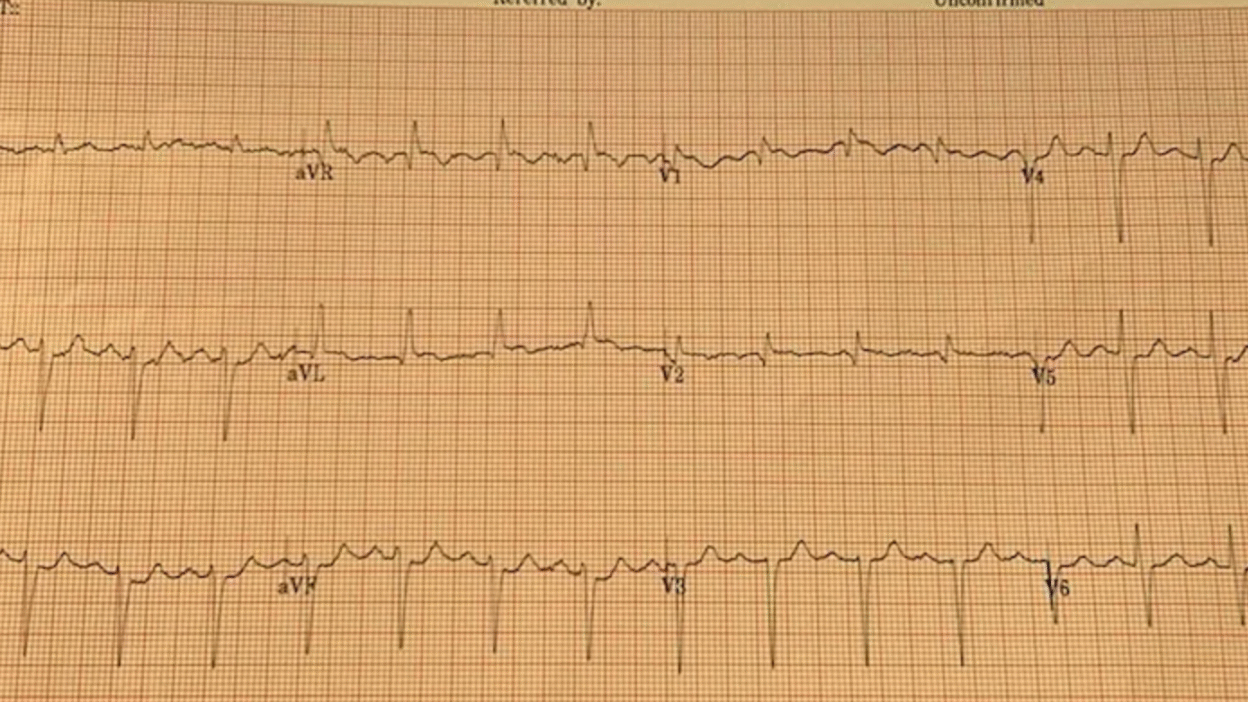
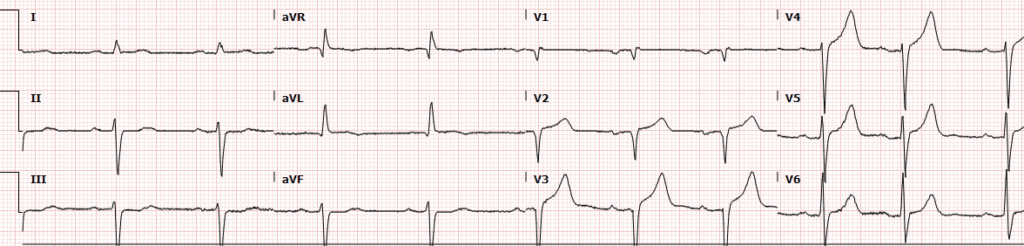
On a weekend evening in a PCI capable centre, triage nurse comes to me with this ECG for a healthy 77 year old with a past history of HTN and BPH who had one hour of acute crushing/squeezing retrosternal chest pain with diaphoresis and nausea.
= = =
= = =
= = =
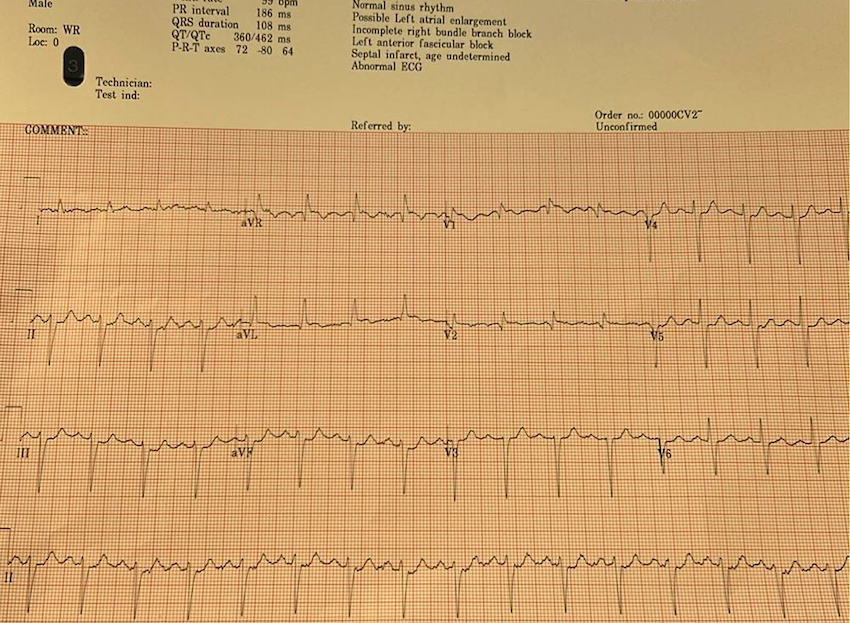
My eyes were drawn to the inferior ST depressions and elevation in aVL concerning for high lateral territory ischemia / South African flag sign. I asked the nurse for serial ECGs q10min and went to immediately assess the patient. Queen of Hearts surprisingly did not detect OMI on this first ECG.
Smith: “inferior” ST depression is NOT inferior “ischemia”. It is reciprocal to high lateral OMI, as recognized by Dr. McArthur.
Inferior ST depression in ACS is reciprocal to ST Elevation in aVL
Isolated inferior ST depression: Not a sign of “inferior ischemia”
Smith: The Queen will probably get this right if the ECG is not cut off (V4-V6 are cut off). You MUST include all 2.5 seconds of EVERY lead. On the other hand, I put it through our most up-to-date algorithm and she said “subendocardial ischemia” with very high confidence. I think SEI is incorrect, but I also do not think that this is South Africa Flag sign. SAF is a pattern due to “Midanterolateral MI,” due to first diagonal occlusion (D1). But you also get high lateral MI from circumflex occlusion. Circ OMI usually has concomitant STD in right precordial leads (concomitant posterior OMI), and there is very subtle precordial ST depression (which is no doubt why the Queen thought SEI). SAF has STE and/or hyperacute T-wave in lead V2, not really present here. So this is more likely posterolateral OMI than midanterolateral OMI.
BUT OMI NEVERTHELESS!! ACTIVATE THE CATH LAB!!
The patient was hypertensive 220/125 and diaphoretic but otherwise stable vitals. He had already chewed 2 baby aspirins at home. Here’s the second ECG:
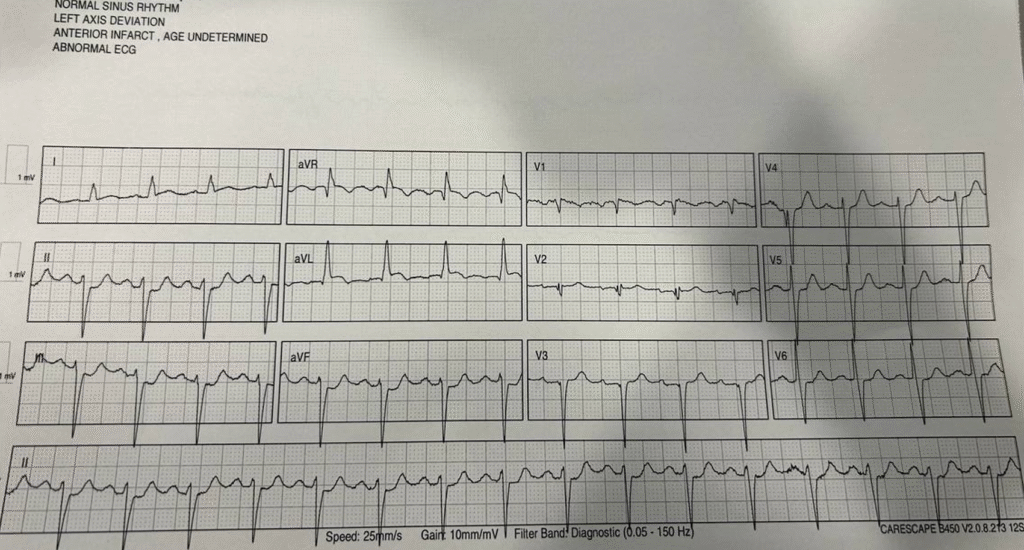
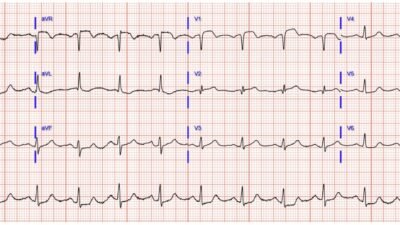
The second ECG obtained at this point, 10 minutes after first, was more obvious with aVL elevated, lead I showing abnormal ST segment morphology, and obvious inferior depressions. QOH recognized this as OMI.
PMcardio for Individuals now includes the latest Queen of Hearts model, AI explainability (blue heatmaps), and %LV Ejection Fraction. Download now for iOS or Android: https://individuals.pmcardio.com/app/promo?code=DRSMITH20. As a member of our community, you can use the code DRSMITH20 to get an exclusive 20% off your first year of the annual subscription. Disclaimer: PMcardio is CE-certified for marketing in the European Union and the United Kingdom. PMcardio technology has not yet been cleared by the US Food and Drug Administration (FDA) for clinical use in the USA.
At this point I started ticagrelor heparin and nitro and called the interventional cardiologist. He agreed with ACS meds but for whatever reason was slightly reluctant about the ECG changes and asked for a repeat ECG first before activating the team.
The next ECG was after nitro, 20 minutes after the second. BP improved to 180 systolic and pain improved to 5/10 from 8/10.
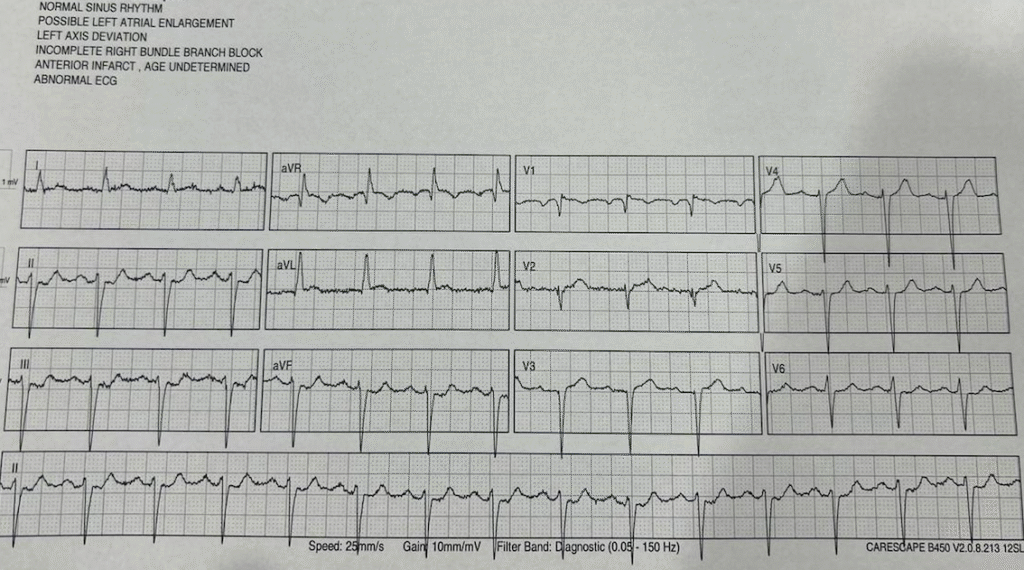
QOH recognized this as OMI as well.
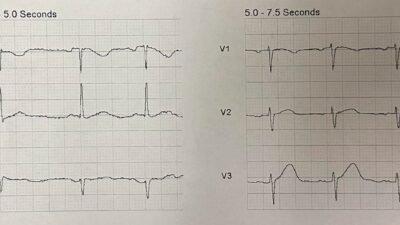
OMI with serial ECGs STEMI negative, first troponin in the normal range
With this ECG the interventional cardiologist stated he didn’t think it met “STEMI criteria” and unconvinced it was acute MI. I advocated that I thought the patient had acute high lateral territory coronary artery occlusion and would benefit from emergent reperfusion. He asked if I would really emergently thrombolyse this patient if I was in a rural centre and I said yes.[like this case] I told him that time is heart and that it would be bad if we delay things and his cath ends up showing high lateral territory occlusion. That seemed to convince him to activate the team. He came down to ED to assess and consent the patient.
THIS is EXEMPLARY CARE by an EMERGENCY PHYSICIAN
As he was getting ready to take the patient to cath lab, the first high sensitivity trop came back at 12ng/L (normal <20). He told me to my face that he wasn’t convinced this was an MI and didn’t really think this deserved cath lab activation. [note: a quarter of patients with true positive STEMI presenting less than 2 hours have initial troponin <99th percentile]
The patient ended up having multi-vessel disease with a culprit 100% occlusion of his distal circumflex which was stented. The cardiologist texted me afterwards saying good call. The next morning trop was 20,782, the echo showed LVEF 60-65% with basal and mid inferolateral RWMA, and ECG showed resolution of ischemic changes with subtle reperfusion TWI in aVL and posterior reperfusion T-waves in V2-V5.
Anyways just wanted to share this good OMI case and appreciate all the work you do around ECGs! For me being early into practice this was an exciting win to convince cardiology and advocate for the patient.
= = =
======================================
MY Comment, by KEN GRAUER, MD (11/11/2025):
Important case submitted by Dr. McArthur (with assistance from Dr. McLaren) that highlights a number of KEY points regarding assessment of acute OMI. These points include awareness that:
- The history should not be ignored.
- Predicting the “culprit” artery may be challenging in the presence of significant underlying coronary disease.
- For as helpful as predicting the “culprit” artery may be — this information is often not essential for deciding on the need for prompt cath.
- Sinus tachycardia, RBBB (Right Bundle Branch Block) and LAHB (Left Anterior HemiBlock) portend important clinical implications when these findings are new and result from an acute event.
- Conditions associated with Brugada phenocopy include acute OMI.
- Availability of a prior ECG will often not be needed to diagnose an acute event — but there are times when access to a prior ECG may prove invaluable for expediting the decision-making process.
= = =
Today’s Initial ECG:
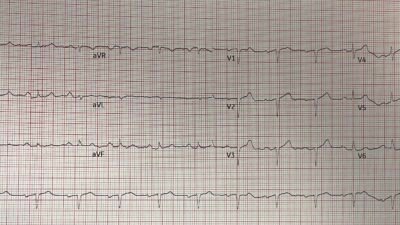
For clarity and ease of comparison — I’ve put the first 2 ECGs in today’s case together in Figure-1. Looking first at today’s initial ECG.
- The rhythm is sinus tachycardia at 100/minute. As emphasized often by Dr. Smith — sinus tachycardia is not commonly seen in uncomplicated acute MI, so its presence should always prompt consideration of other factors (ie, heart failure, a more extensive infarction, other complications — such as the hypertensive urgency with initial BP = 220/125 mm Hg in today’s case).
- There is RBBB/LAHB in ECG #1 (QR pattern in V1 with wide terminal S wave in V6 — with predominant negativity in the inferior leads indicating marked left axis deviation).
- Although the QRS complex is of small amplitude in leads V1,V2 — there are definite Q waves in these leads — with a QS complex in neighboring lead V3.
- Although subtle (because of small QRS size) — there is ST elevation in both leads V1 and V2 (RED arrows in these leads).
- ST elevation is also clearly seen in lead aVL — with reciprocal ST depression in each of the inferior leads (BLUE arrows in leads II,III,aVF).
- Finally — there is no more chest lead ST elevation, but instead ST segment flattening in leads V3,V4,V5 (with slight ST depression in V4,V5).
IMPRESSION: Given the very worrisome history that this previously healthy 77-year old man presents with ( = sudden onset of “crushing” CP [Chest Pain] with diaphoresis and nausea) — this initial ECG has to be interpreted as indicative of an acute OMI until proven otherwise!
- Finding a prior ECG on this patient would be invaluable — because IF the sinus tachycardia, RBBB/LAHB, anterior Q waves (with QS in lead V3) and ST elevation in leads V1,V2 and aVL are all new — this would suggest acute proximal LAD occlusion with extensive acute infarction. Regardless (as per Drs. McArther and McLaren) — prompt cath is indicated.
- The above said — it turns out that the “culprit” artery in today’s case was not the LAD — but the distal LCx in this patient with significant underlying multi-vessel disease. While true that ST elevation in high-lateral lead aVL often signals LCx or marginal branch occlusion — ST elevation is also regularly seen in lead aVL with proximal LAD occlusion (which is why access to a prior ECG would be so helpful in today’s case, by telling us if the RBBB/LAHB that so often accompanies acute LAD occlusion is new or was previously present, perhaps from a prior “silent” event in this 77-year old man who was not known before to have coronary disease).
- LCx OMI is often associated with lateral chest lead ST elevation — but none is seen here. On the contrary, there is ST depression in leads V4,V5 — which is what might be expected in association with acute LAD occlusion in a patient with underlying multi-vessel disease. That said — none of this matters, because regardless of the identity of the “culprit” artery in today’s case — the need for prompt cath with PCI is immediately indicated by the worrisome history and the initial ECG.
= = =
Figure-1: Comparison between the initial ECG — and the repeat ECG done ~10 minutes later. (To improve visualization — I’ve digitized the original ECG using PMcardio).
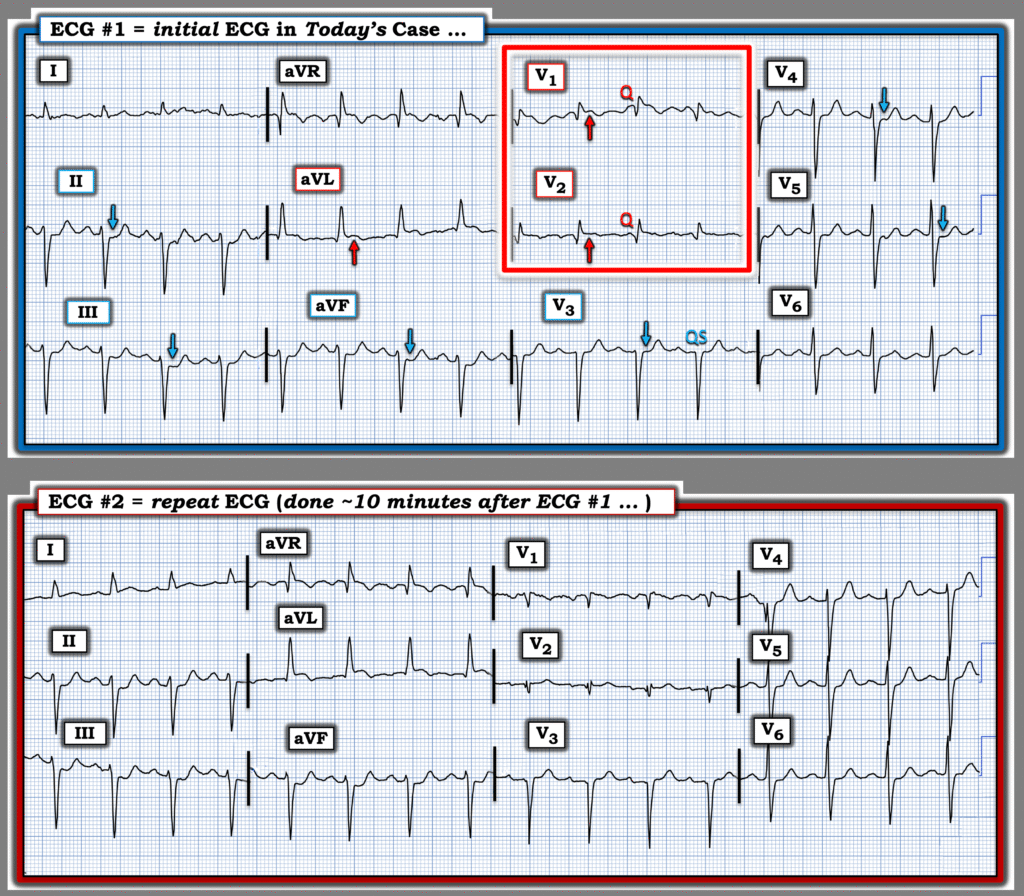
= = =
The Repeat ECG — done 10 minutes later …
My thoughts on lead-to-lead comparison of these first 2 ECGs in Figure-1 were the following:
- Sinus tachycardia at 100/minute is still present in the repeat ECG.
- The main difference between ECG #1 and ECG #2 — is that RBBB is no longer present. Instead, the slightly elevated ST segment in lead V1 now manifests a much slower decline to the baseline that resembles a Brugada ECG pattern (clearly not nearly enough ST elevation to qualify as a Brugada-1 pattern — but with similarity in “shape” of the ST segment). As I review in My Comment in the January 13, 2025 post — ischemia and/or infarction have been associated with Brugada phenocopy. Any resemblance to phenocopy was gone with the final ECG in today’s case done the following morning — but it is good to be aware that acute ischemia is among potential causes of Brugada phenocopy (with this ECG pattern often resolving without consequence after treatment).
- Otherwise — apart from slightly more ST elevation with beginning T wave inversion in lead aVL — and perhaps slightly less ST depression in leads V4,V5 — I did not see significant ST-T wave changes between these 2 tracings.
= = =
= = =