Written by Jesse McLaren
A 40 year old developed sudden chest pain radiating to the jaw,
with diaphoresis and vomiting. What do you think?
What do you think?
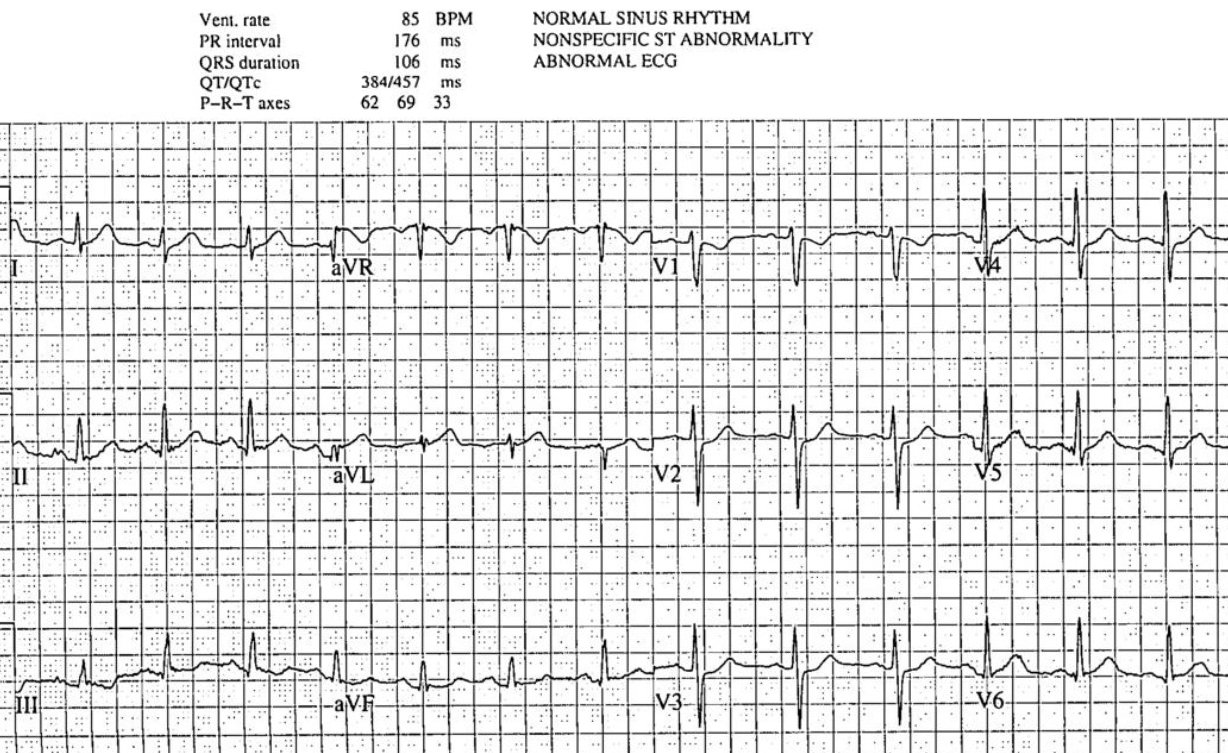
There’s normal sinus rhythm with normal conduction, normal axis,
normal R wave progression and normal voltages. There are hyperacute T wave in I/aVL
and possibly V5-6, with reciprocal change in III. There’s also ST depression in
V1-3. The computer interpretation labeled this ECG as “nonspecific”, and it
does not meet STEMI criteria. But there are ischemic abnormalities in the
majority of leads that add up to an ECG diagnostic of posterolateral Occlusion
MI.
The paramedics had previously done an ECG and were concerned
about posterior MI, so they administered ASA/nitro and took the patient
directly to the cath lab. The patient arrived at the CCU with 90 minutes of ongoing
chest pain, where the ECG above was recorded followed by a 15-lead ECG 4
minutes later.
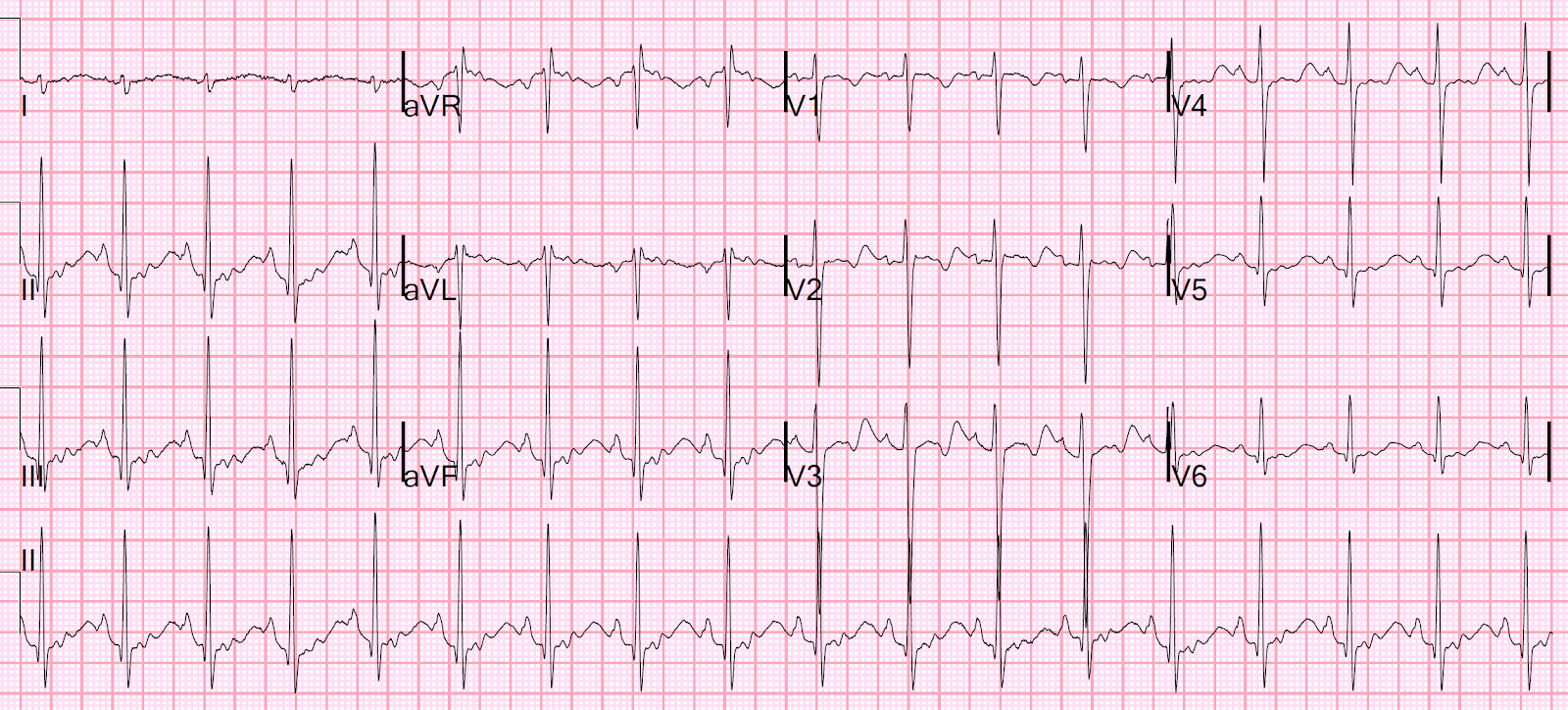
There’s now an acute Q wave and convex ST segment in aVL,
and mild ST elevation in the posterior leads V8-9, again confirming
posterolateral OMI. But the cardiologist did not think it met STEMI criteria, so
the patient was sent to the emergency department for assessment.
The patient arrived in the ED with ongoing chest pain and
another ECG was obtained:
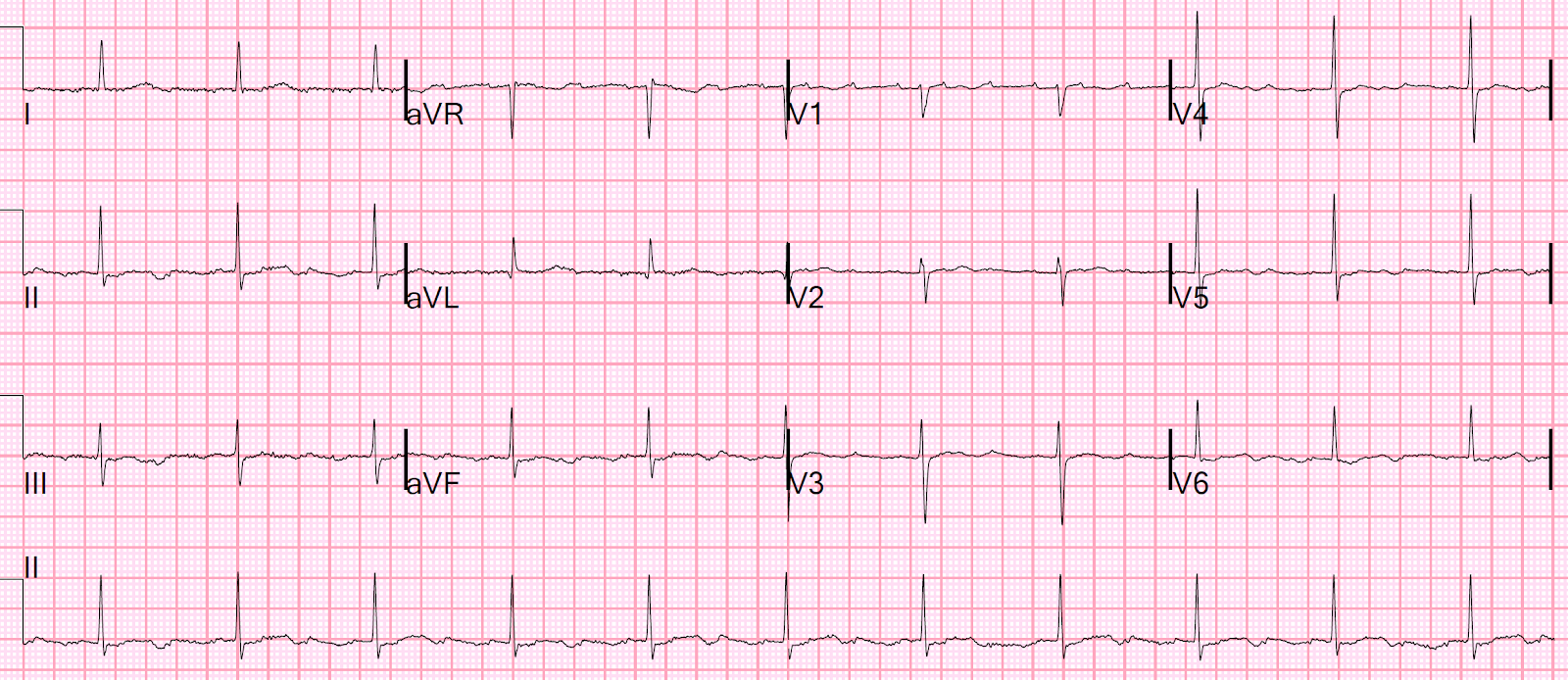
There’s now mild ST elevation in I/aVL and deeper ST
depression in V3, confirming ongoing posterolateral OMI. The emergency
physician gave more nitro and called a Code STEMI.
Cardiology assessed the patient in the ED with serial ECGs
over the next hour, during which time the chest pain continued and the first high sensitivity troponin I returned at 100 ng/L (normal <26).
There’s ongoing posterolateral OMI, in a patient with refractory
chest pain and positive troponin. But cardiology felt this did not consistently
meet STEMI criteria, and noted the pain was reproducible on palpation and
unrelieved by nitro, so they administered morphine and asked for a CT chest to
rule out dissection. But reproducible or other forms of atypical chest pain do not exclude myocardial
infarction in a patient with such a high pre-test likelihood; morphine is
associated with delayed reperfusion; and refractory ischemic chest pain is an
indication for the cath lab even in the absence of ECG changes.
The CT found coronary calcifications but no aortic
dissection. The patient was still in pain, with a repeat troponin of 400 ng/L.
Cardiology reassessed the patient and diagnosed NSTEMI, treated with
ticagrelor/heparin/nitro and non-urgent cath. Because of refractory chest pain
the patient received multiple doses of morphine for another couple of hours in
the emergency department, and then was taken to the cath lab. Angiogram found a
99% occlusion of the first obtuse marginal, as predicted from the first ECG, with TIMI-1 flow.
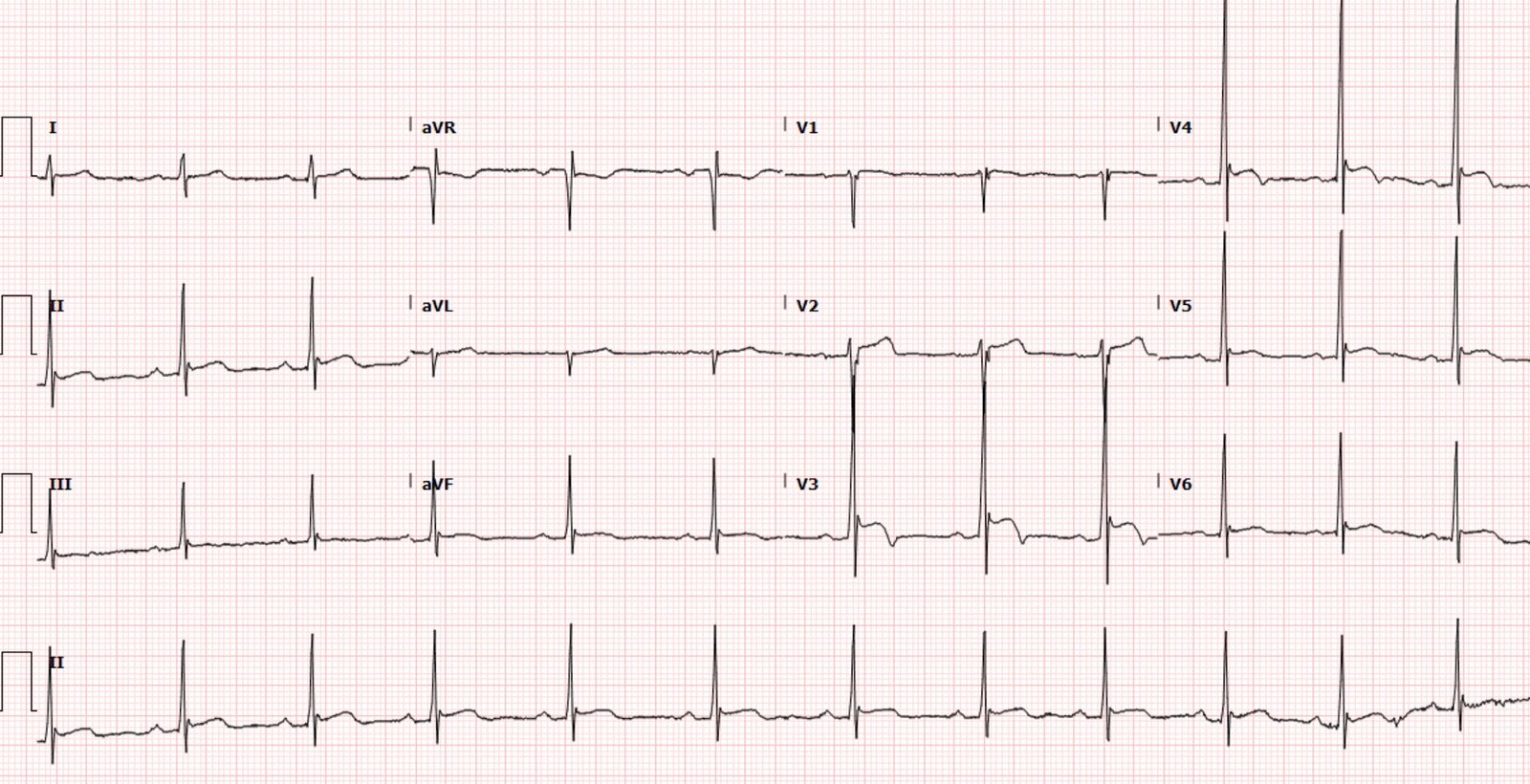
Below is the next day ECG:
T waves in I/aVL/V6 have deflated to their normal size and
developed reperfusion T wave inversion, with ongoing Q wave in aVL, and taller
T waves in the anterior leads from posterior reperfusion.
Peak troponin was 50,000 ng/L and the echo found an EF of 50%
with basal/lateral wall motion abnormalities. The discharge diagnosis was “NSTEMI,” implying that the cath lab activation was false and the cancellation was appropriate.
False/inappropriate activation or cancellation?
There are many
studies on false positive and inappropriate activation of the cath lab. “False positive
activation” is a retrospective term for patients whose angiogram reveals no
culprit lesion.(1-7) But this is a heterogeneous group, including “appropriate
false-positive activation” (including STEMI mimics like takotsubo, coronary
spasm or myocarditis, which can only be diagnosed after angiogram has ruled out
acute coronary occlusion) and “unnecessary cath lab activation” (erroneous ECG
interpretation and MI ruled out).(8) Unfortunately, “inappropriate activation”
has come to mean any cath lab activation that is canceled by the cardiologist
because the ECG didn’t meet STEMI criteria. This defines “appropriateness” by the surrogate marker of STEMI criteria, not the actual outcome of acute coronary occlusion. If the cath lab is activated for STEMI(+)NOMI (e.g non-ischemic elevation from LVH or early repol, with a non-occlusive lesion with TIMI-3 flow) this could still be classified as appropriate; yet cath lab activation for STEMI(-)OMI (i.e does not meet STEMI criteria but does have an occluded artery) could be considered inappropriate. Many
studies have excluded “inappropriate activation” patients from further
analysis, and gone onto classify the “appropriate activations” as true positive
STEMI vs false positive STEMI. (9-11) But what about false negative STEMIs, false cancellations and
inappropriatecancellations, like our patient above?
According to the
STEMI paradigm, there’s no such thing as a false negative STEMI, because this
is by definition “Non-STEMI”—even though 25-30% of NSTEMI have a totally occluded
artery and double the mortality.(12) This is what Smith/Meyers have termed the
“no false negative paradox.” Even those studies that have looked at outcomes of
“inappropriate activation” have denied the possibility of false negative STEMI.
Mixon et al. have a figure that illustrates this:
According to this classification, activations based on STEMI
criteria are deemed appropriate and classified as either true positive or false
positive based on the angiogram, but activations without STEMI criteria are by
definition inappropriate and false positive regardless of angiogram findings—even
though 41% of this group was subsequently diagnosed as “NSTEMI/UA”, a
heterogenous group including STEMI(-)OMI, NOMI and unstable angina.(13) Shamim
et al. found 5 of 62 “inappropriate activations” (8%) required emergent cath
but all were diagnosed with “Non-STEMI.”(14) Similarly, Degheim et al found 2
of 37 cancellations (5.4%) were “erroneously cancelled and found to be actual
STEMIs when patients underwent coronary angiography later for unresolved chest
pain and positive biomarkers.”(15) But these patients were not “actual STEMIs”, they
were STEMI(-)OMIs.
If false negative
STEMI doesn’t exist, then neither does inappropriate cath lab cancellation—even
if it results in patient death like this case or this case. There is only one study on
inappropriate cath lab cancellation, but this was defined as ECGs that met
STEMI criteria, in a patient with typical chest pain ≤12
hours, who had no contraindications but was not taken for cath, and ruled in
for MI.(16)
Only 1% of cancellations met this narrow definition, but 18% were canceled
because of “ST-T changes not consistent with STEMI,” 25% were canceled because of
bundle branch block or paced rhythm, and 10% required PCI. How many of these were
inappropriate cancellations, with ECGs diagnostic of STEMI(-)OMI?
“Appropriate
cath lab activation” should be based on the patient outcome that benefits from emergent
reperfusion—acute coronary occlusion—and criteria for cath lab activation
should be expanded beyond classic STEMI criteria to incorporate the latest advances.(17)
This now include evidence-based ECG criteria that can identify OMI and
differentiate it from mimics,(18) which is twice as sensitive as STEMI criteria
with preserved specificity (19). The STEMI paradigm has restricted its quality
metrics like door-to-balloon time to STEMI(+)OMI, but we need to apply them to
all patients with acute coronary occlusion including STEMI(-)OMI.(20). If we
use balancing measures like false or inappropriate activation to monitor and prevent against
over-activation of the cath lab, shouldn’t we also track false or inappropriate
cancellation to monitor and prevent delayed reperfusion?
Take home:
1.
Primary ST depression V1-4 is posterior OMI
until proven otherwise. A 15 lead may or may not show ST elevation, but can also show dynamic anterior and inferior/lateral changes
2.
Lateral OMI can be subtle but can often be
identified by acute Q waves, subtle ST elevation, convex ST segments,
hyperacute T waves, and inferior reciprocal change
3.
Reproducible chest pain does not rule out OMI in
high pre-test patients, and morphine is associated with delayed reperfusion
4.
Rather than restricting “Code STEMIs” to
STEMI(+)OMI, appropriate cath lab activation needs to be expanded to include
ECG signs of STEMI(-)OMI and clinical signs like refractory ischemia
5.
When the cath lab is canceled for lack of STEMI
criteria, but patients are subsequently found to have an acute coronary
occlusion, this should not be labeled “NSTEMI” with “appropriate cancellation”
but STEMI(-)OMI with inappropriate cancellation
References:
1. Larson. ‘False-positive’ cardiac
catheterization laboratory activation among patients with suspected ST-segment
elevation myocardial infarction. JAMA 2007
2. Yougquist et al. A comparison of
door-to-balloon times and false-positive activations between emergency
department and out-of-hospital activation of the coronary catheterization team.
Acad Emerg Med 2008
3. Nfor. Identifying false-positive
ST-elevation myocardial infarction in emergency department patients. J of Emerg
Med 2012
4. Chung et al. Characteristics and
prognosis in patients with false-positive ST-elevation myocardial infarction in
the ED. Am J Emerg Med 2013
5. Groot et al. Characteristics of patients
with false-ST-segment elevation myocardial infarction diagnoses. Eur Heart J
2016
6. Shoaib et al. Impact of pre-hospital
activation of STEMI on false positive activation rate and door to balloon time.
Heart, Lung and Circ 2022
7. McCabe et al. Prevalence and factors
associated with false-positive ST-segment elevation myocardial infarction
diagnoses at primary percutaneous coronary intervention-capable centers: a
report from the Activate-SF Registry. Arch Intern Med 2012
8. Kontos et al. An evaluation of the accuracy
of emergency physician activation of the cardiac catheterization laboratory for
patients with suspected ST-segment elevation myocardial infarction. Ann Emerg
Med 2010
9. Lu et al. Incidence and characteristics
of inappropriate and false-positive cardiac catheterization laboratory
activations in a regional primary percutaneous coronary intervention program.
Am Heart J 2016
10. Boivin-Proulx et al. Effect of real-time
physician oversight of prehospital STEMI diagnosis on ECG-inappropriate and
false positive catheterization laboratory activation. CJC Open 2021
11. Garvey et al. Rates of cardiac
catheterization cancelation for ST-segment elevation myocardial infarction
after activation by emergency medical services or emergency physicians: results
from the North Carolina Catheterization Laboratory Activation Registry. Circ
2012
12. Khan et al. Impact of total occlusion of
culprit artery in acute non-ST elevation myocardial infarction: a systematic
review and meta-analysis. Eur Heart J 2017
13. Mixon. Retrospective description and
analysis of consecutive catheterization laboratory ST-segment elevation
myocardial infarction activations with proposal, rationale, and use of a new
classification scheme. Circ Cardiovasc Qual Outcomes 2012
14. Shamim et al. Electrocardiographic
findings resulting in inappropriate cardiac catheterization laboratory
activation for ST-segment elevation myocardial infarction. Cardiovasc Diagn
Ther 2014
15. Degheim et al. False activation of the
cardiac catheterization laboratory: the price to pay for shorter treatment
delay. JRSM Cardiovasc Dis 2019
16. Lange et al. Cancellation of the cardiac
catherization lab after activation for ST-segment-elevation myocardial
infarction: frequency, etiology, and clinical outcomes. Circ Cardiovas Qual
Outcomes 2018
17. Rokos et al. Appropriate cardiac cath lab
activation: optimizing electrocardiogram interpretation and clinical
decision-making for acute ST-elevation myocardial infarction. Am Heart J 2010
18. Aslanger, Meyers, Smith. Recognizing
electrocardiographically subtle occlusion myocardial infarction and
differentiating it from mimics: ten steps to or away from the cath lab. Turk
Kardiyol Dern Ars 2021
19. Meyers et al. Accuracy of OMI ECG
findings versus STEMI criteria for diagnosis of acute coronary occlusion
myocardial infarction.
20. McLaren, Meyers, Smith, et al. From
STEMI to occlusion MI: paradigm shift and ED quality improvement. CJEM 2021