Written by Emerson Floyd MD (Edits by Grauer, Meyers, Smith)
Smith: Isn’t it sad that we need to be psychologists in order to try to persuade interventionalists to do the right thing?
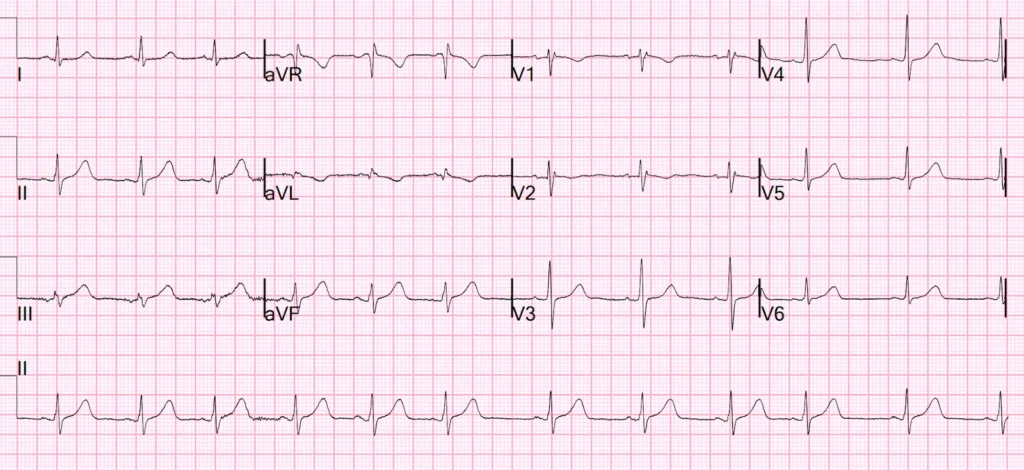
Emerson begins the CASE: I was shown the following ECG at 0345 am while I was walking down the hall to another patient’s room.
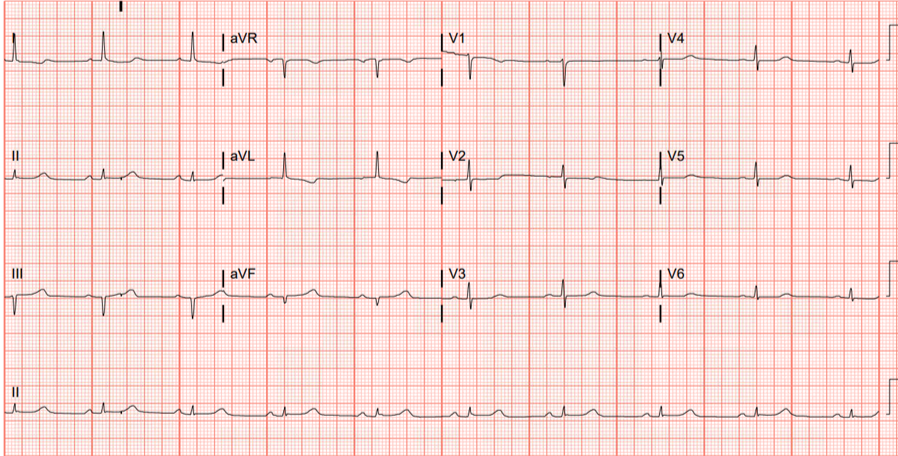
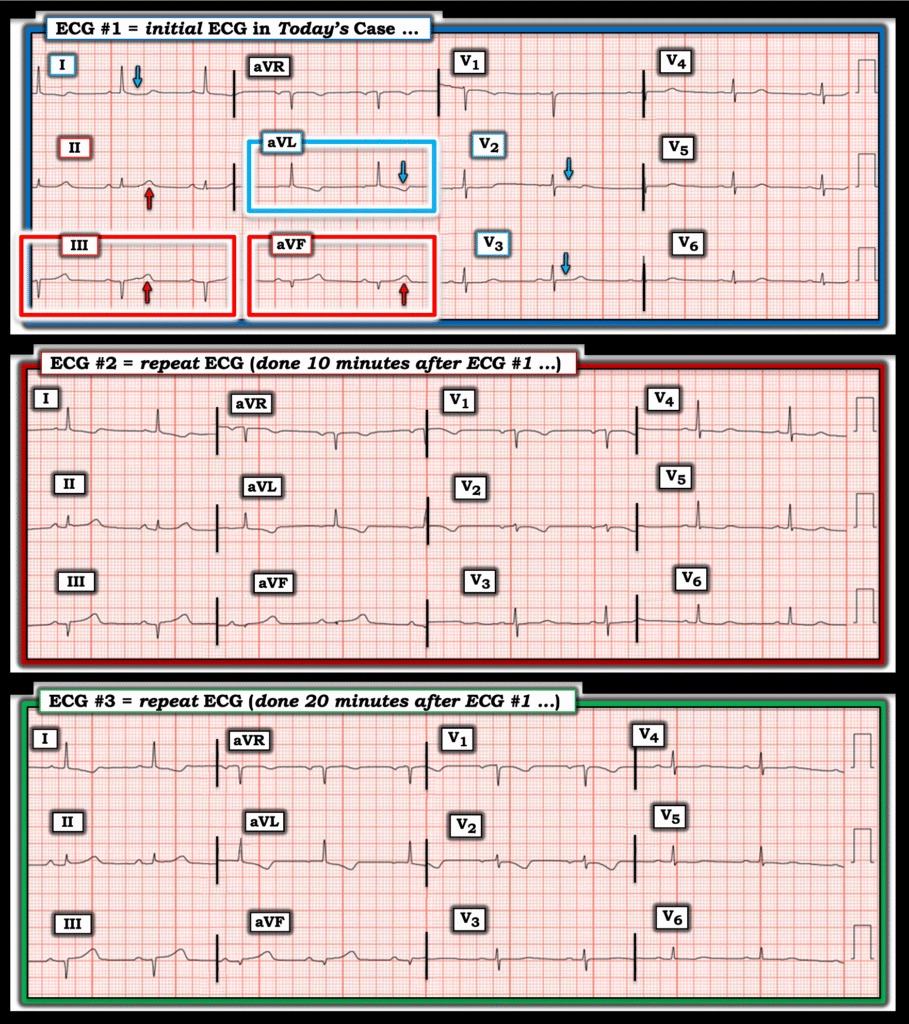
My suspicion for acute coronary occlusion was immediately raised by this initial ECG. Lead aVL was the most concerning lead, and along with leads I and V2 — a clear picture of inferior injury with high lateral and “posterior” reciprocals painted.
(As an aside, the first time I remember seeing such an ECG was at a talk delivered by Dr. Stephen Smith that I attended my Intern year. Many subtle inferior OMIs. I recall getting a one-word response when I asked a smart senior resident what he thought about the talk: “Terrifying.” But I since learned, and now teach the residents I work with — that reciprocal changes often manifest before injury to the infarcted territory in the limb leads.)
Smith: what makes this ECG difficult is not whether it represents infarction (MI) or not (it clearly does), but whether it is an ACUTE OMI: an old inferior MI can show Q-waves like this with some persistent ST Elevation (LV Aneurysm). But when there are inferior Q-waves, inferior aneurysm and acute inferior OMI are much more difficult to differentiate than Anterior OMI from Anterior LV Aneurysm. In this case, as Emerson will discuss, the T-wave is too large (bulky) to be old.
The Queen knows it:
Emerson continues: I dropped what I was doing and went straight to this patient’s room. Upon seeing her I was further convinced that she was experiencing an acute coronary occlusion: The best I can describe her appearance is of some pallor with a stoic, uncomfortable but fearful look.
The patient told me that she had woken up from sleep at 2 am, with chest pain radiating to her left shoulder that had since become constant. She just had a history of lymphedema and did not smoke, did not have DM or HTN and did not take daily meds.
I told the patient that I was highly suspicious that she was having a heart attack and that the most important treatments were revascularization and aspirin (the latter she had already gotten). I said that I anticipated activating the cath lab, but I was not sure that an interventional cardiologist would take her based on her initial ECG.
At that point — I did a point of care ultrasound, which showed an EF of over 50% and no obvious wall motion abnormality (Possibly there was some inferior hypokinesis — but I was not certain of this). I told the patient we would be getting our next two ECGs 10 minutes apart.
To Emphasize: I had mixed feelings about my strategy. I was already convinced that this patient needed cardiac catheterization — and therefore told myself that I would be activating the cath lab within the next half-hour regardless of what serial ECGs showed. Part of me wanted to activate immediately.
- The above said — I had the benefit of a bedside encounter with the patient to inform her of my suspicion.
- I was at a community site without residents or fellows.
- I wanted to be able convince the interventional cardiologist, who I was waking up at 4 am, to take this patient to the lab before they had even seen the patient.
- I feared that the interventional cardiologist would recommend serial troponins if I activated immediately just based on this ECG. This could really hurt the patient.
- In addition — with chest pain starting at 2 am, it’s possible that a troponin drawn at 330 am could be negative, delaying her care by another 3 hours. Over that amount of time she would be at risk of not only losing a significant amount of myocardium — but also of sudden death due to arrythmia.
- I thought of a similar case I was familiar with — which presented with this ECG (which I show below, courtesy of Mubarak Alhatemi):
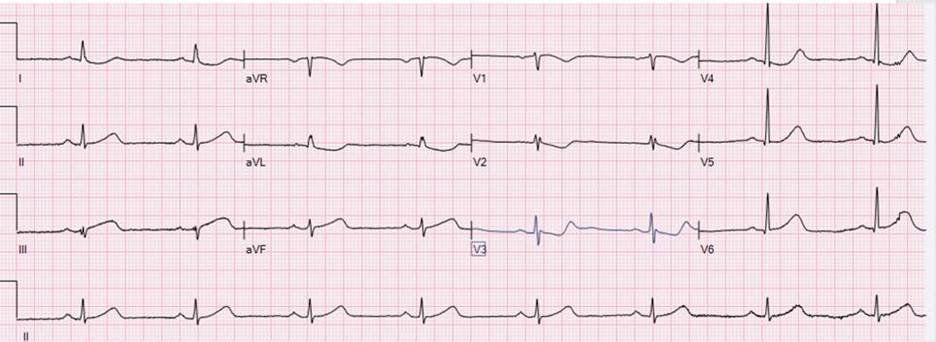
Smith: This ECG from Mubarak Alhatemi’s case is not subtle. Instead — it is 100% diagnostic of acute infero-postero (and lateral = V6) OMI.
Smith: Here is a much more subtle ECG, but still diagnostic for inferior posterior OMI: 30-something woman with a HEART score of zero, EDACS of 2, computer “Normal” ECG, and initial troponin < Limit of Detection
The Queen even knows this one:
New PMcardio for Individuals App 3.0 now includes the latest Queen of Hearts model and AI explainability (blue heatmaps)! Download now for iOS or Android. https://www.powerfulmedical.com/pmcardio-individuals/ (Drs. Smith and Meyers trained the AI Model and are shareholders in Powerful Medical.)
Today’s CASE Continues:
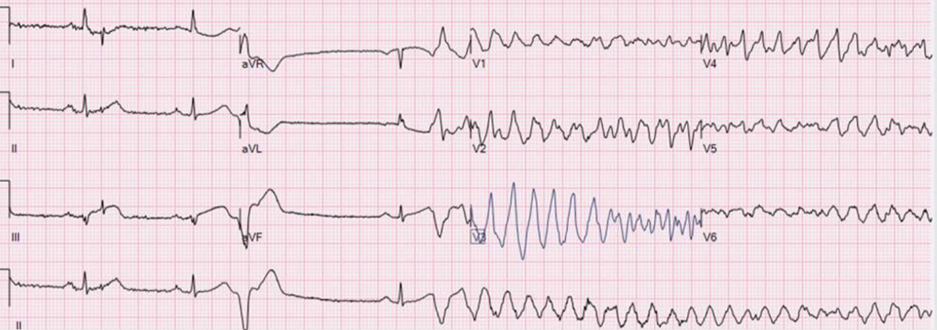
Emerson: The reason I thought of the above case from Mubarak Alhatemi — is because of what soon followed in that case — which was this rhythm below that was recorded while the patient was still connected to the ECG machine! Considering this abrupt onset of VFib in that case — I knew for today’s case, that I needed to find the best strategy in a “world of STEMI criteria” — in order to maximize myocardial salvage and minimize risk to my patient.
Today’s CASE Continues:
10 minutes after ECG #1 was recorded — a repeat ECG was recorded:
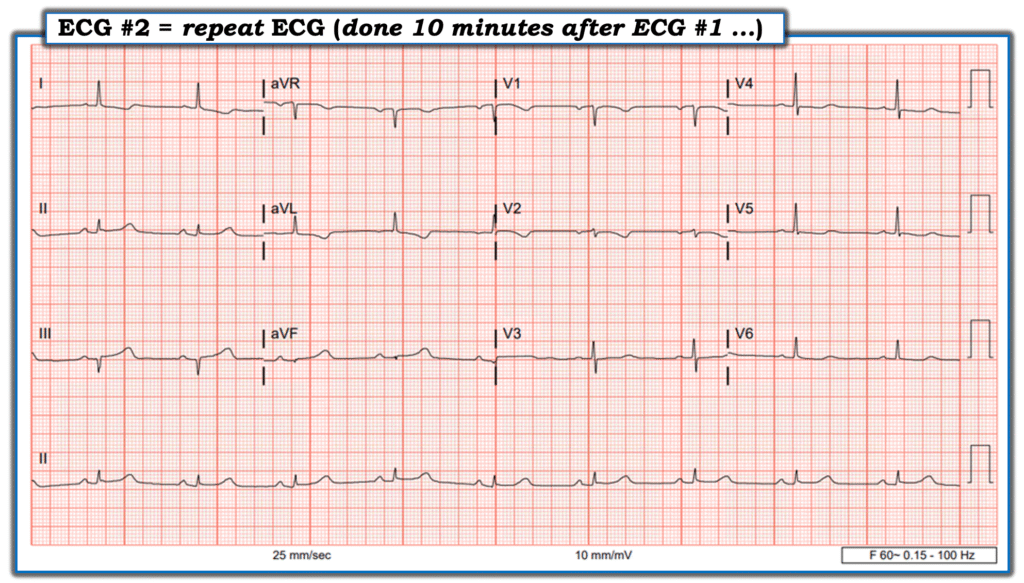
To my “eye” — ECG #2 represented evolution during the 10 minutes since ECG #1 was recorded. In regards to leads III and aVF, the ratio of the QRS to the area under the T-wave was increasing. This “proportionality” is a foundational concept in defining hyperacute T-waves (based on the work of Smith and Meyers). I observed that in lead III — I could now even fit the diminished QRS complex into the area under the T-wave ( = a heuristic I teach to junior trainees as the “easiest way to identify a potentially hyperacute T-wave).
Smith: this rule of thumb is not totally accurate because amplitude (height) is only one feature of a hyperacute T-wave. It is important as far as it contributes to the total area under the curve of the T-wave. We have shown quantitatively that it is area under the curve relative to QRS amplitude AND symmetry which define HATW. Area under the curve is dependent on: 1) QT interval 2) height (amplitude) and 3) straightness of the upslope and downslope of the T-wave. Here is our abstract, with images.
Case continued: I was able to confirm that the patient’s chest pain was still present, but somewhat improved after morphine.
Smith: it is dangerous to give morphine. Morphine is associated with worse outcomes in OMI because it hides the ongoing ischemia. Do not use it until you are committed to the cath lab!!
At that moment — I was called away to deal with a time-sensitive clinical situation. I asked for the patient to remain connected to the ECG machine for one more tracing, to be obtained after 10 more minutes. I returned to the room at that time to review this last ECG #3 — with this to be the last piece of data I observed before immediately activating the cath lab:
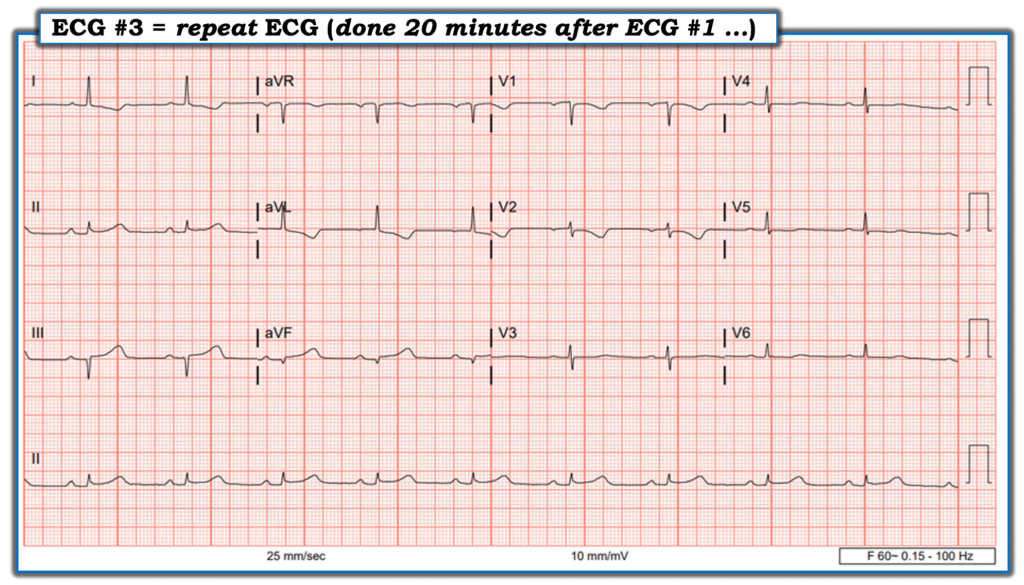
ECG #3 nearly met STEMI criteria in II, III and avF. The high-lateral T wave inversions were deepening. The lead V1 and V2 electrodes were probably still placed too high on the chest — but still were consistent with “posterior” injury.
I activated the cath lab — and immediately thereafter called the Interventional cardiologist. “Thanks for taking my call, previously healthy 71 -year old woman with an hour and a half of chest pain to the left shoulder — on serial ECGs evolving inferior acute coronary occlusion, now borderline STEMI positive with high lateral and anterior reciprocals. I think she may have some inferior hypokinesis. I’m convinced this patient is having an inferior wall Type-1 event, and needs to go to the lab.”
The interventional cardiologist agreed — and the patient was whisked away to the cath lab. I had activated the cath lab for this patient within 30 minutes after I first met her — and she was gone 5 minutes later.
- The 1st Troponin result came back right after the patient had gone to the cath lab. It was normal.
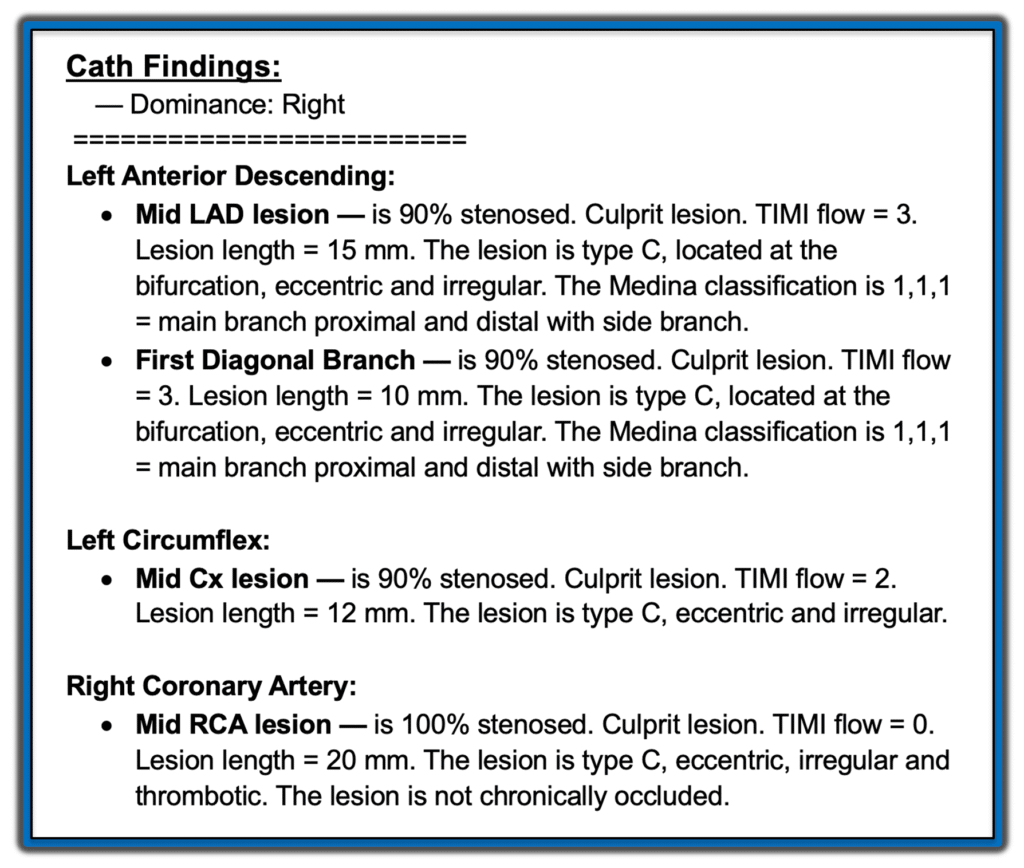
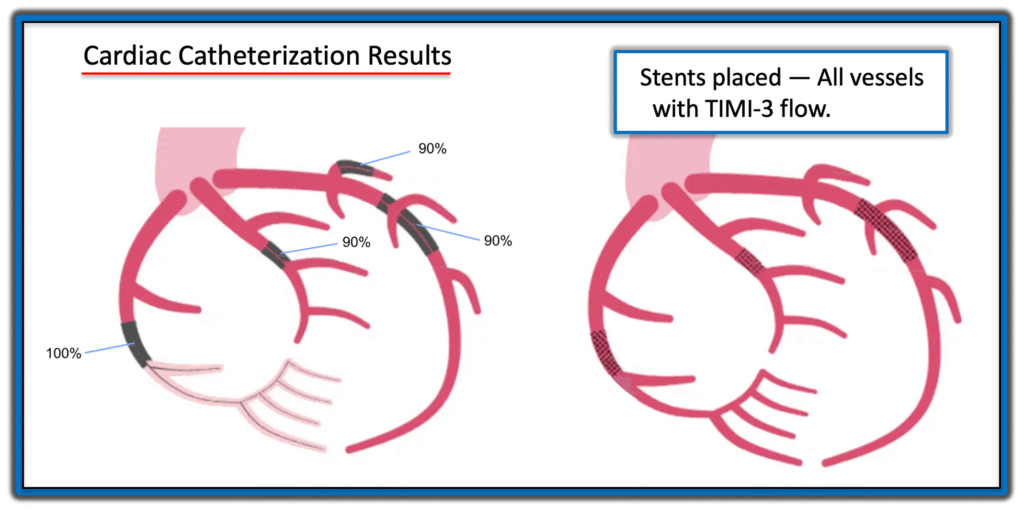
Results of Cardiac Catheterization:
In a technically challenging 2 hour procedure the following findings were obtained:
Additional Data:
Formal Echocardiogram, done the next day — demonstrated a 50-55% ejection fraction, with Grade 1 diastolic dysfunction, normal wall thickness and hypokinesis of the inferior basal and inferior basoseptal walls.
- The patient had a similar EF and a good functional status on Echo repeated one month later.
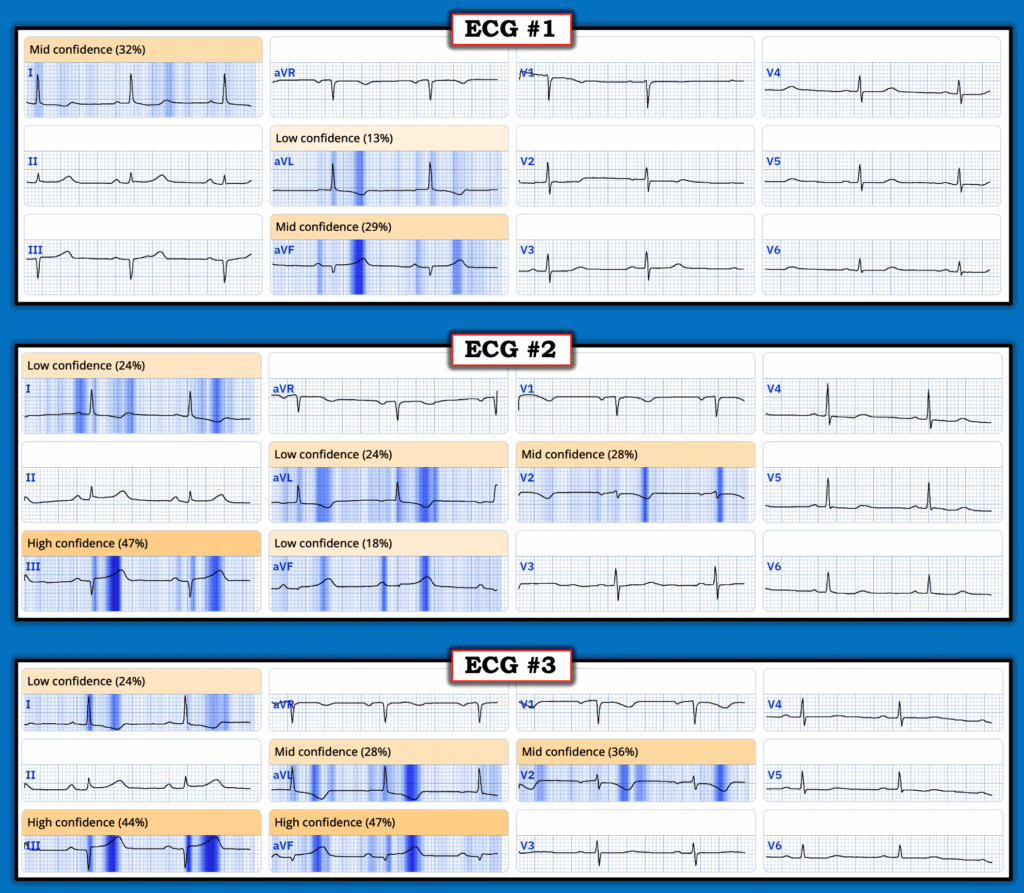
QOH (Queen of Hearts) Assessment:
While I was managing this case — it did not occur to me to ask the Queen of Hearts what she thought — perhaps because I already had my own convictions about what was happening clinically. I was building a case that I was going to pursue no matter what. But I asked QOH the next day — and was not surprised that she expressed increasing confidence with the serial ECGs:
Final Reflections on this CASE:
As an Emergency Physician, especially one who works mostly at night — I feel like I have one best shot to get the patient the revascularization that they need. Even when I’m convinced that a patient needs revascularization, the best course of action varies based on the particular case. It may be in my medicolegal favor to activate the lab as soon as I’m convinced that the patient needs a stat cath — but it may not necessarily be in the patient’s favor. This is becauase the interventional cardiologist, perhaps not convinced by the ECG — might ask for serial troponins — or even ask for the patient to be heparinized and admitted to their service where the patient will be evaluated for cath at some later time …
Sure, I could reactivate the cath lab if the patient met STEMI criteria on subsequent serials — but there’s never any guarantee that the injured area will evolve to the point of satisfying millimeter-based STEMI criteria on the ECG. And even if they do — this might not be for several (or more) hours later. The 2022 ACC recommendations note that hyperacute T-waves are “STEMI equivalents” — but there is no universal agreement on what a “hyperacute T-wave” is at the current time.
Smith: As above, we now know quantitatively what is a hyperacute T-wave
Even with the courage of my clinical convictions, it is a challenging but rewarding task to get my patients the best care I can under the flawed STEMI paradigm — and to teach trainees to do the same. While having an ECG that meets STEMI criteria in association with symptoms consistent with ACS may remain the patient’s most fail-safe ticket to the cath lab — I encourage my residents to develop eyes for “proportions and reciprocals” in identifying ischemia on the ECG, rather than counting millimeters of ST elevation.
While I expect the OMI paradigm to continue to gain favor over the STEMI paradigm — I do not expect the Emergency Physician’s job to ultimately change, that is — To form a conviction about a patient based on a history, physical and collected data, and to then take the right actions to get that patient the care they need as soon as possible. Serial ECGs will often be necessary — and ECG interpretation skills will always be needed to recognize when they are necessary.
======================================
MY Comment, by KEN GRAUER, MD (7/16/2025):
I found today’s case by Emerson Floyd laudable — in that it highlights the insights gained by an emergency physician through his internship and residency, and now in practice as an Attending physician, passing on the knowledge he gained to the residents he teaches. What at first seemed like such very subtle ECG signs — is now instantly recognized by Dr. Floyd as an OMI in progress.
- I love the wisdom in this quote from Dr. Floyd — “I encourage my residents to develop eyes for proportions and reciprocals in identifying ischemia on the ECG, rather than counting millimeters of ST elevation.” The flawed STEMI paradigm counts millimeters. That’s why it misses more than 1/3 of all OMIs — and why even among those infarctions that are recognized by the STEMI paradigm, it often takes many more hours after the OMI paradigm has already identified the ongoing event for sufficient millimeters of ST elevation to develop.
- I thought Dr. Floyd’s approach to today’s case is a model to be emulated. He immediately became highly suspicious of an acute-OMI-in-progress within seconds of seeing ECG #1.
- He knew the “fastest path” for his decision making — was to spend a quick minute with the patient. The patient’s appearance alone confirmed a worrisome history of CP (Chest Pain) — which given Dr. Floyd’s high suspicion of acuity from the initial ECG meant that regardless of whatever might follow (ie, even if the 1st and 2nd Troponins were negative — or the chest pain improved — or the next ECG did not show any change) — regardless of what might follow, this patient was in need of prompt cath.
- All that remained was to find the fastest path toward being able to convince the interventional cardiologist in the middle of the night that cardiac cath should be done as soon as this would be possible.
How Did Dr. Floyd Know Cath was Needed So Quickly?
To facilitate assessment — I’ve put today’s 3 tracings together in Figure-1.
- Among the principles Dr. Floyd applied in his rapid assessment of today’s initial ECG — was to look to see if there was any one or two leads that he instantly knew were abnormal in a patient with CP. The 2 leads within the RED rectangle in ECG #1 immediately caught my “eye”. As is often the case in the inferior leads — QRS amplitude is not large (the QRS is tiny in lead aVF).
- Applying Dr. Floyd’s “proportions” — There should be no doubt that the T wave in lead aVF is disproportionately enlarged = hyperacute (ie, This T wave in aVF being “fatter”-at-its-peak and wider-at-its-base than expected given tiny size of the QRS in this lead).
- Applying the principle of neighboring leads — Given the hyperacute T wave in lead aVF — the T wave in lead III is also disproportionately enlarged (and to a lesser extent — the T wave in the 3rd inferior lead = lead II — is also larger-than-it-should-be given tiny size of the QRS in lead II).
- Confirmation of an acute inferior OMI is then forthcoming from application of Dr. Floyd’s “reciprocals” — as the ST-T wave in lead aVL manifests the mirror-image opposite picture from the hyperacute ST-T wave in lead III.
- As we often emphasize on these pages — there usually is a common blood supply to the inferior and posterior LV walls — such that I always look for evidence of posterior OMI when I suspect inferior OMI (and vice versa). Here, the principle is that normally there should be slight, gently upsloping ST elevation in leads V2 and V3 — yet we see shelf-like flattening if not slight depression of the ST segment in both of these leads. This essentially confirms acute posterior OMI until proven otherwise.
To Emphasize: At this point — Dr. Floyd knew the cath lab needed to be activated. But in the interest of optimizing his chance of convincing a cardiologist at 4 am to take a patient to cath who this cardiologist had not yet seen — Dr. Floyd thought it worth another 20 minutes to record 2 serial tracings that would hopefully remove any possibility of doubt by the cardiologist who was still at home.
- The changes in Figure-1 that we see in the 2 ECGs that followed are somewhat subtle, but definitely present.
- KEY Point: Isn’t it much easier to appreciate serial ST-T wave changes between ECGs #1, 2 and 3 — when we look lead-by-lead with each tracing right next to the other? It is all-too-easy to overlook serial ECG changes when ECGs are not compared lead-to-lead.
- It should be clear from lead-to-lead comparison of the 3 tracings in Figure-1 — that the degree of ST-T wave abnormality progressively increases from ECG #1 — to ECG #2 — to ECG #3. The interventionist agreed with Dr. Floyd’s assessment — and total time from patient arrival in the hospital until arrival in the cath lab was no more than 35 minutes!
- And — the 1st Troponin was normal! (but this is not unusual — and fortunately this did not matter! ).
==============================
Figure-1: Comparison of the 3 serial ECGs in today’s case.
==============================