Written by Pendell Meyers, edits by Steve Smith
A man in his 60s with history of hypertension and MI 10 years ago, with PCI, presented to an outside hospital complaining of chest pain that started while mowing the lawn. His chest pain was located in the central chest, non-radiating, and associated with diaphoresis, nausea, and vomiting.
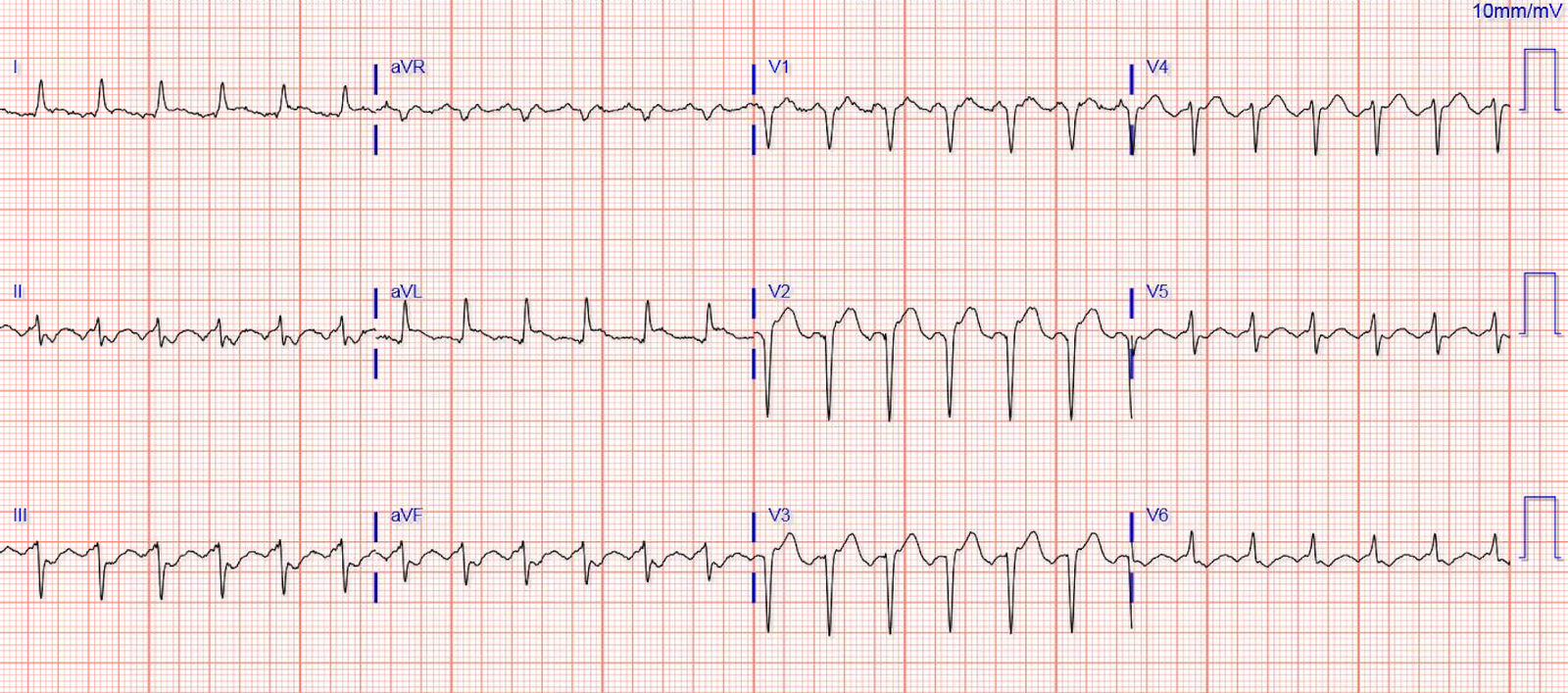
Here is his ECG on arrival:
 |
| What do you think? |
This ECG is all but diagnostic of subepicardial ischemia of the anterior, lateral, and inferior walls, most likely due to Occlusion MI (OMI), probably of the LAD. This is evidenced by hyperacute T-waves in leads V3-V6. Leads II and aVF also have hyperacute T-waves. The R-wave progression is extremely poor which is also common in large anterolateral Occlusion MI (OMI). There is a very small amount of STE in some of the anterior, lateral, and inferior leads which do NOT meet STEMI criteria.
This ECG is highly suspicious for LAD OMI. If you have any doubts, you should record another one every 5 minutes until you are convinced.
The most likely location for an occlusion producing OMI changes in the anterolateral and inferior leads is the proximal-to-mid LAD. Usually this will be either a type II LAD (which supplies the apex) or a type III LAD (which goes even further and curves around the apex to the inferior aspect of the apex).
Remember that the ECG reports what is happening to the myocytes, then you must use that information to make inferences about what the patient needs. Acute coronary occlusion is the most common and most treatable cause of this pattern, but it is not the only cause. The myocytes do not know why they are dying, they can only report their death and hope you can see it and figure it out. Takotsubo, spasm, low flow with a preexisting stable coronary lesion, etc. are other causes of acute focal myocyte death which produce the same ECG findings.
This patient clearly needs emergent angiography and reperfusion of the LAD lesion that is causing his Occlusion MI until proven otherwise.
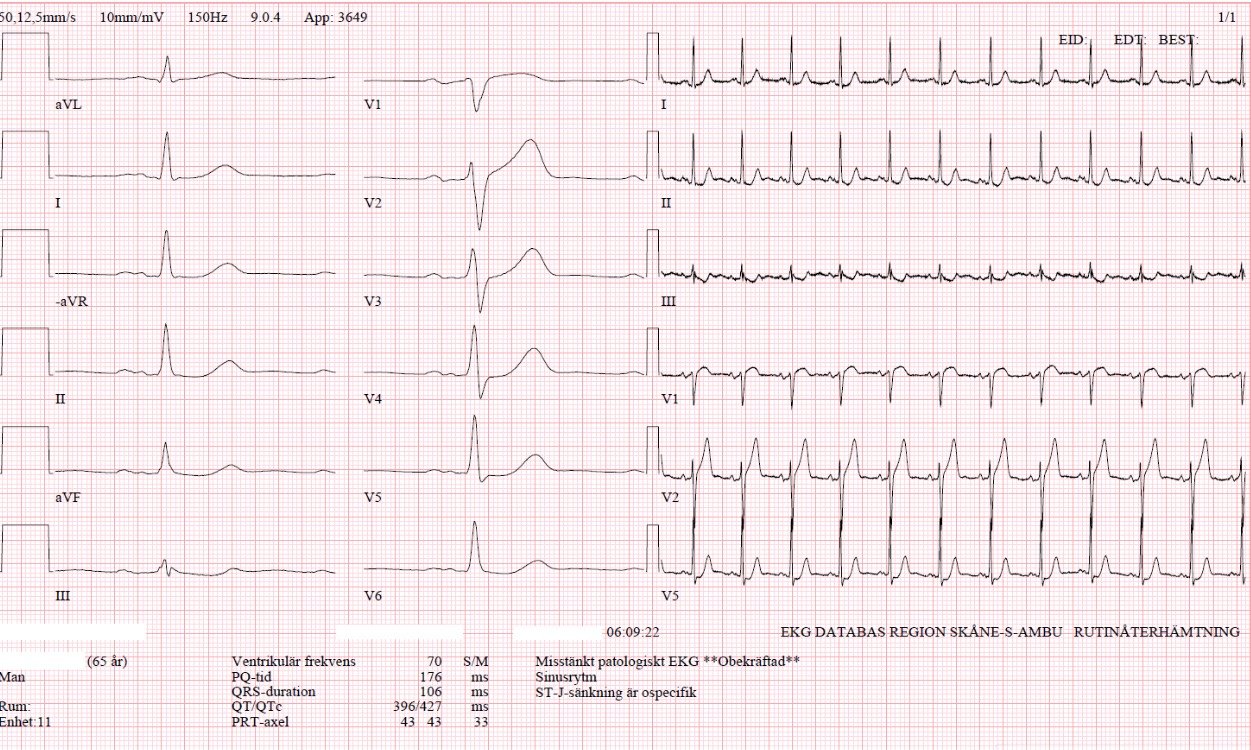
Here is his prior ECG on file from the outside hospital from long ago:
The EM physicians at the outside hospital activated the cath lab transfer protocol (unless cardiology cancels the activation, our cath lab is activated, and the patient is transferred immediately from the outside hospital to our cath lab).
The case was reviewed by all parties, and it was stated correctly that the ECG does not meet the STEMI criteria.
Cath lab activation was cancelled but the transfer was accepted for urgent cardiology evaluation.
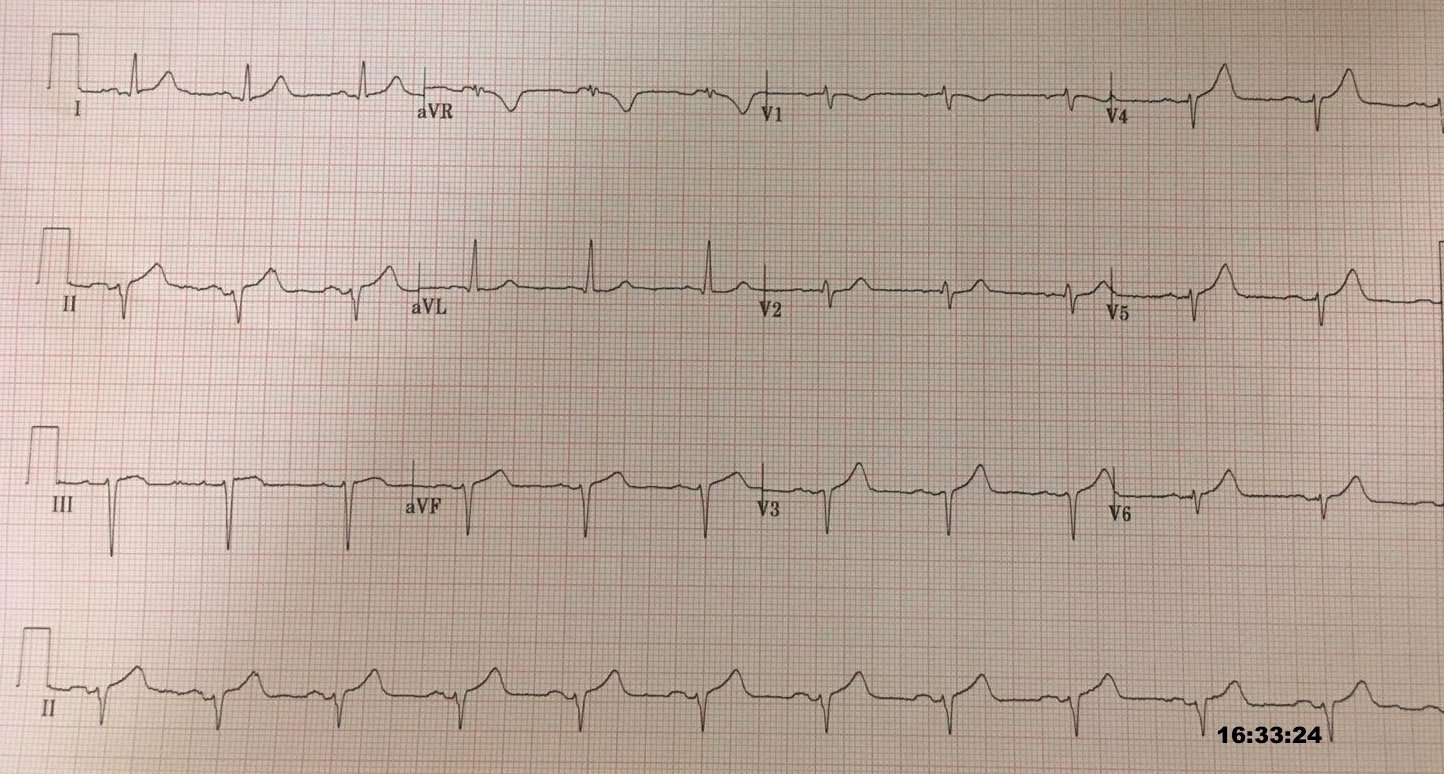
40 minutes after the first ECG, just before transport, a repeat ECG was obtained:
 |
| Continued, active OMI of the anterior, lateral, and inferior walls. |
In the ambulance during transport, the patient suddenly suffered VF arrest.
He was defibrillated immediately and had return of normal mental status.
Upon arrival, providers again reviewed the ECGs and found no reason on ECG for emergent catheterization. Given the VF arrest during transport, however, they appropriately all agreed that cath should happen sooner rather than later.
They took him almost immediately for catheterization. They found an acute lesion of the LAD at the site of the prior stents, including 70% proximal LAD lesion and 95% mid-LAD stenosis with TIMI 3 flow at the time of cath. There was also a chronic total occlusion of the RCA. The LAD lesion was acute and required 3 stents to restore flow. Here are the images of the acute LAD lesion:
 |
| The mid-LAD is nearly occluded, but with distal flow. |
 |
| In this view it appears to be a total occlusion, however the video shows delayed slow flow through the LAD lesion. |
 |
| Mid-intervention. |
 |
| Post-intervention. |
Troponin T peaked at 3.86 ng/mL (very high).
Echo the next day showed EF 35% with wall motion abnormalities of the mid-anterolateral, mid-anterior, mid-inferior, mid-inferolateral, mid-inferior, mid-anterior septum, mid-inferior septum, anterior apex, lateral apex, inferior apex, septal apex, and the apical cap. This distribution matches the distribution of OMI findings on ECG (although some of the WMA could be explained by the previous Q-wave infarct in the prior ECG).
Here are his repeat ECGs after intervention:
 |
| Similar findings. |
Learning Points:
Patients deserve to have their Occlusion MI recognized prior to (or even in the total absence of) STEMI criteria.
Expert ECG interpretation could have prevented this man’s cardiac arrest, and almost certainly would have resulted in a much smaller MI and therefore better long term prognosis.
The STEMI vs. NSTEMI paradigm is not the best way to decide who needs emergent reperfusion therapy. The most recent available RCT evidence (the FTT Meta-analysis published in 1994) would suggest that this patient receives mortality harm from emergent reperfusion therapy (thrombolytics), because his ECG does not meet STEMI criteria (see diagrams below representing the mortality effects of thrombolytics from the FTT meta-analysis). This is obviously false, and this demonstrates how the STEMI vs. NSTEMI paradigm has brainwashed us into failing to recognize other ECG findings which predict Occlusion MI. In the FTT meta-analysis there was no expert ECG interpretation, there was simply an undefined trichotomous classification of “ST elevation,” “ST depression”, or “normal” ECG. Additionally the studies in the FTT meta-analysis were done in an era without cardiac catheterization, and thus they were unable to distinguish between occlusions and non-occlusions among those without ST elevation. For more, see The OMI Manifesto.
———————————————————–
Comment by KEN
GRAUER, MD (9/21/2018):
———————————————————–
I
would suggest the following alternative title for this blog post = “A
difficult and unfortunate way to learn” … And, there is a lot to learn
in this case:
- First
— The
wrong question was asked … The
question apparently asked by decision-makers was, “Is there ECG evidence of an
acute STEMI”. Instead, in a patient like this with new-onset chest pain — the question that should have been asked is, “Does this initial ECG suggest an acute
process?” — because if it does, then urgent cath to define the anatomy
is clearly indicated. - Somewhere
along the way — the initial ECG was misinterpreted.
It’s hard to tell from the information available where this mistake occurred. The
EM physicians at the outside hospital appropriately activated the cath lab
transfer protocol — but that order was subsequently negated by others … So
while true that strict ECG criteria for acute STEMI were not met — the “correct
interpretation” of this initial ECG given the clinical context of new-onset chest pain should have been
precisely as described above by Dr. Meyers = hyperacute T waves in multiple
leads, in association with slight ST
segment elevation + poor R wave progression (actually, loss of r wave from V2-to-V3). - The
correct
interpretation of this initial ECG (which I’ve reproduced in Figure-1) should have included
the clinical
correlation that the above described
abnormal ECG findings in multiple leads are highly suspicious for acute
LAD occlusion ( = OMI), with clear indication for immediate
cath and coronary reperfusion. - When multiple
leads all show abnormal findings — then
the findings you are seeing are probably real. So despite the
fact that strict criteria for acute STEMI are not met in the TOP tracing in Figure-1
— hyperacute T waves (defined as taller, broader and
fatter-than-expected with respect to the QRS complex) are seen in leads I, II, aVF, and V3-V6. Among the remaining
leads — there is some ST elevation in lead III — and, an inappropriately
straight ST segment with overly prominent T wave in V2. In addition, lead aVR
shows an unexpectedly deep and broad inverted T wave (doubtlessly reflective of a reciprocal change to the upright hyperacute
T waves in multiple leads). This makes for no less than 10 out of 12 leads on this initial ECG that are
obviously abnormal. When 10 leads show a consistent theme of ECG
abnormalities — then these ECG
findings must be taken as real.
—————————————
Additional
lessons to be learned in this case are suggested by comparison with the prior
ECG (BOTTOM tracing in Figure-1).
- As
per Dr. Meyers — We have no idea as
to when in this patient’s history the prior ECG was obtained. We suspect this
tracing was obtained during reperfusion of this patient’s previous MI — but the
meaning of the distinctly biphasic T waves in V2,V3 — with T inversion in V4,V5
might portend a very different clinical significance depending on whether
infarction had just happened, was in the process of resolving, or had happened
several years earlier. - Regardless
of when this prior ECG was obtained — the learning point is that IF
there had been any doubt about the acuity of the initial ECG — comparison of
the 2 tracings in Figure-1 should confirm that the changes in the initial ECG
are definitely acute and strongly suggestive of acute OMI.