A 79-year-old woman with a history of hypertension and a benign brain tumor was awakened by chest pain and dyspnea. She reported having similar but transient symptoms over several months, but on this occasion, her symptoms persisted. She contacted her primary care physician who referred her to the emergency department due to ST-segment depression (initial ECG unavailable). Vital signs in the ED were as follows: BP 159/87 mmHg, oxygen saturation 99%, RR 19/min, and she was afebrile. The below ECG was recorded. What do you think?
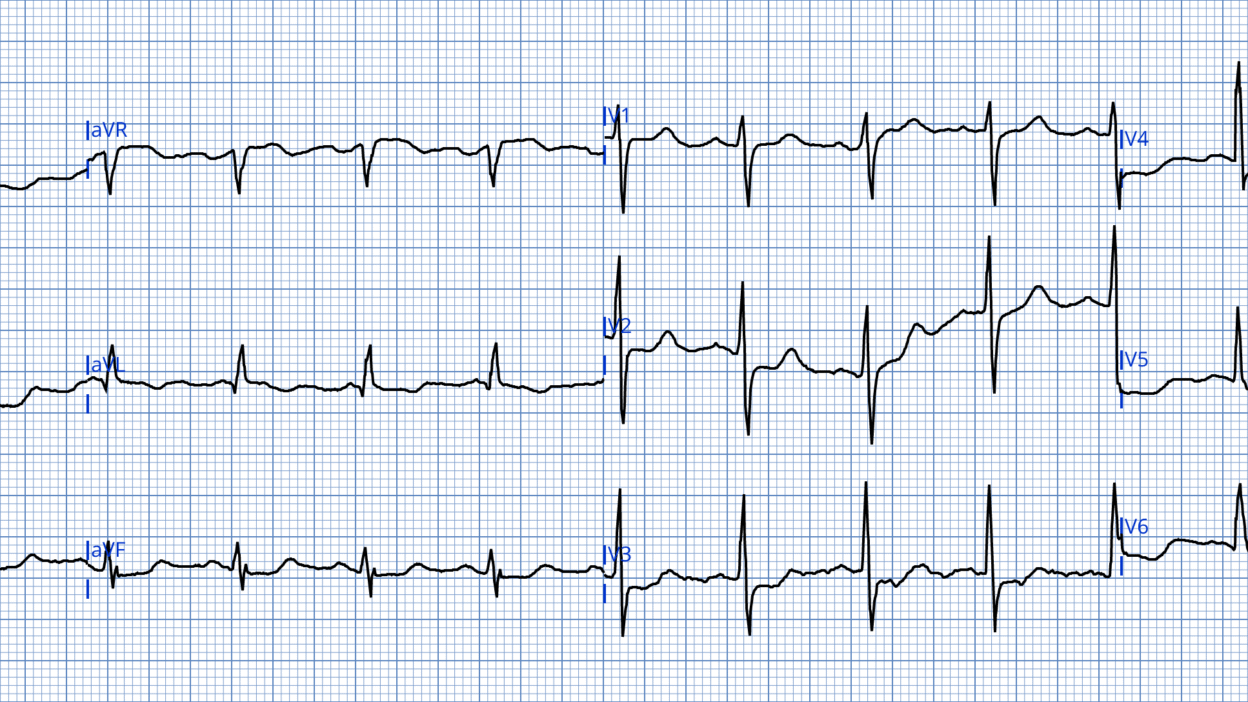
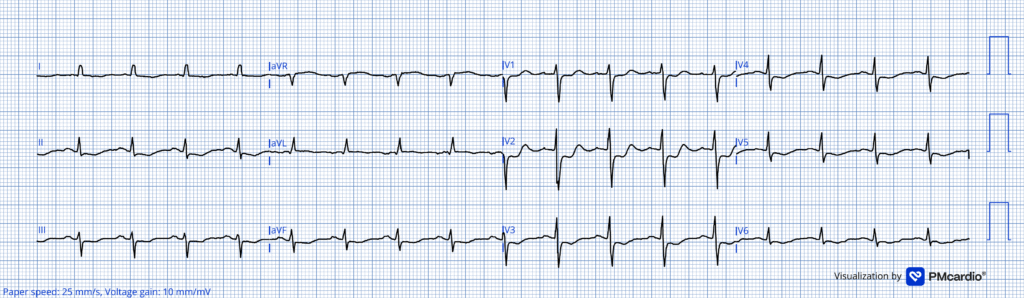
ECG #1
What do you think?
Smith: I would say this is a posterior and high lateral OMI, strongly suggestive of circumflex occlusion (STD max V3-4; STD inferior reciprocal to STE in aVL), so one should activate the cath lab emergently. I have not read further yet and am interested to know what is coming.
Magnus continues:
There is sinus rhythm with a heart rate of about 100 bpm. QRS complexes are narrow with ST-segment depression in leads II, III, aVF, and V3-V6. (Lead V2 also likely has ST depression, though baseline artifact makes this difficult to asses properly).
As emphasized repeatedly on this blog, ST depression due to subendocardial ischemia does not localize and cannot identify the specific ischemic territory. When evaluating ST depression in the context of ACS it is important to differentiate OMI from subendocardial ischemia as the causes and treatment can differ. Also it is important to remember that SEI and OMI may be present in the same ECG. Aslanger pattern is an example of this.
In general, the presence of tachycardia is more suggestive of a supply/demand imbalance (type II MI) rather than focal occlusion, unless the ejection fraction is severely reduced or mechanical complications is present.
Smith: this is very mild tachycardia, at approximately 102 beats per min.
Back to the case: The patient received morphine for pain relief, with reported symptom improvement. It is unclear if she became pain free. Initial high-sensitivity troponin I returned elevated (2280 ng/L, ref <16). She was admitted with a working diagnosis of NSTEMI.
Smith: morphine is terrible if you are not committed to the cath lab.
Magnus: Whenever there is inferior ST depression it is important to examine high lateral leads, as subtle ST elevation here may be a clue to lateral OMI. The ST segment in aVL in ECG #1 is shows very slight STE, which in the context of widespread ST depression, should alert the provider to the possibility of lateral OMI being present. If I had cared for this patient I would have repeated the ECG to assess for dynamic changes and attempted a better-quality recording, especially of lead V2. Also, I would have withheld morphine as it may mask ongoing symptoms, complicating clinical assessment.
If symptoms persist despite optimal therapy, urgent angiography is indicated regardless of whether the ST depression represents OMI or subendocardial ischemia from ACS.
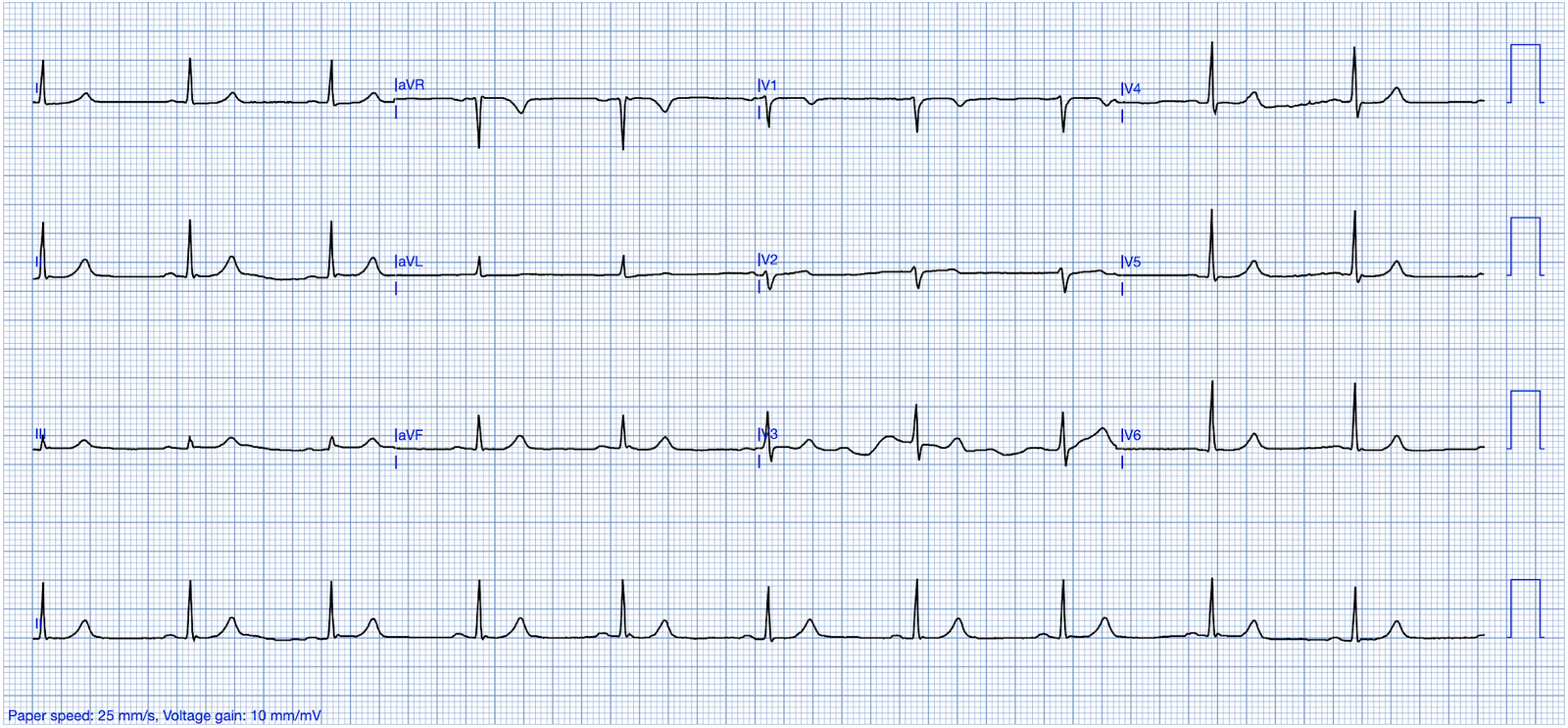
ECG #2
A follow-up ECG obtained the next morning showed sinus tachycardia and persistent ST depression, now clearly maximal in V2. Drs. Meyers and Smith demonstrated that maximal ST depression in V1-V4 in acute coronary syndrome is highly specific for OMI. The specificity of ischemic STD max V1-V4 was 97% for OMI. Ischemic STD maximal in leads V1-V4 is OMI until proven otherwise, in contrast to STD maximal in V5-6 which is usually SEI (non-occlusive ischemia). There is one exception to this general rule: In atrial fibrillation with RVR, the SEI without OMI often manifests as maximal STD in V1-4.
The patient eventually underwent coronary angiography, which revealed diffuse (triple vessel) coronary disease and a subtotal LCx occlusion. Peak troponin I reached 18.911 ng/L (ref < 16ng/L). Echocardiography showed a posterolateral wall motion abnormality. Despite the delayed treatment this patient did well. At cath there was a subtotal occlusion and it is likely that there was some reperfusion-reocclusion during her hospital stay with the reperfusion episode(s) minimizing myocardial damage.
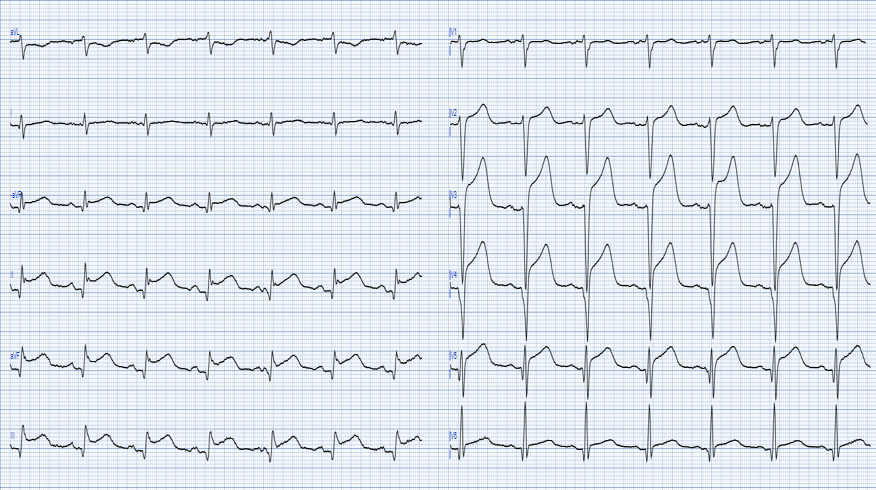
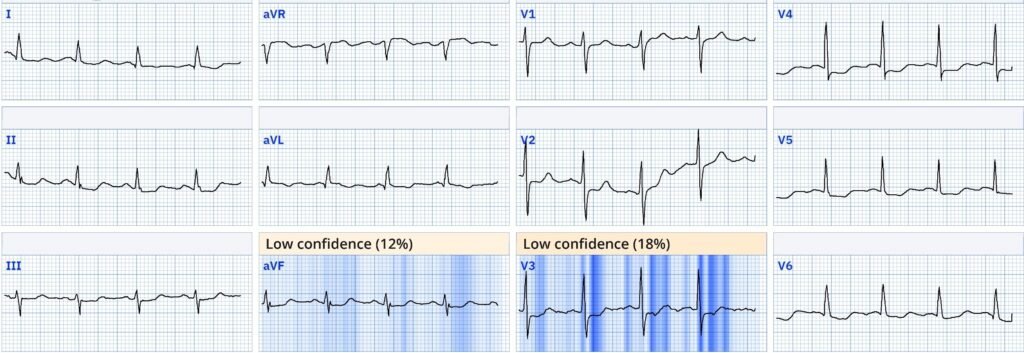
How did the Queen of hearts interpret the initial ECG? The AI model flags both ECGs as OMI. This is interesting, as she is not influenced by the tachycardia and does see OMI pattern through the diffuse ST depression of SEI (which is clearly also present).
Explainability ECG #1
Explainability ECG # 2
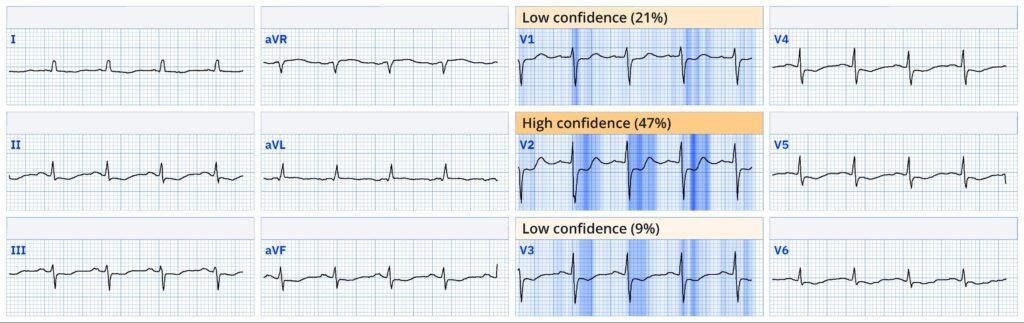
See this recent case for an example on how the Queen of Hearts evaluates ST depression in V1-V4 as not OMI.
PMcardio for Individuals now includes the latest Queen of Hearts model, AI explainability (blue heatmaps), and %LV Ejection Fraction. Download now for iOS or Android: https://individuals.pmcardio.com/app/promo?code=DRSMITH20. As a member of our community, you can use the code DRSMITH20 to get an exclusive 20% off your first year of the annual subscription. Disclaimer: PMcardio is CE-certified for marketing in the European Union and the United Kingdom. PMcardio technology has not yet been cleared by the US Food and Drug Administration (FDA) for clinical use in the USA.
Learning Points
- Relief or persistence of symptoms is key for ongoing management; the use of morphine may mask symptom progression.
- Differentiation between OMI and subendocardial ischemia on ECG can be challenging and sometimes both processes overlap.
- Even if it is SEI (and not OMI), if there are persistent ischemic symptoms refractory to Nitroglycerine, the patient needs the cath lab emergently.
- All pain will respond to morphine, even if there is ongong ischemia and infarction, so don’t give morphine until committed to emergent cath lab.
- ST depression in inferior or precordial leads requires close scrutiny of high lateral leads and possible repeat ECGs to detect dynamic changes.
Opiates are associated with worse outcomes in Myocardial Infarction.
See this case: A man his 50s with chest pain. What happens when you treat with morphine rather than with reperfusion?
—-See this study showing an association between morphine and mortality in ACS:
Use of Morphine in ACS is independently associated with mortality, at odds ratio of 1.4. Meine TJ, Roe M, Chen A, Patel M, Washam J, Ohman E, Peacock W, Pollack C, Gibler W, Peterson E. Association of intravenous morphine use and outcomes in acute coronary syndromes: Results from the CRUSADE Quality Improvement Initiative. Am Heart J. 2005;149:1043–1049.
And Another that we wrote:
—-Bracey, A. Meyers HP. Smith SW. Wei L. Singer DD. Singer A. Association between opioid analgesia and delays to cardiac catheterization of patients with occlusion Myocardial Infarctions. Academic Emergency Medicine 27(S1): S220; May 2020. Abstract 556.
Main result: STEMI(-) OMI Patients
65 (23.9%) patients were found to have STEMI(-) occlusion myocardial infarction (OMI) at the time of cardiac catheterization. The 45 patients with STEMI(-) OMI without pre-cath opioids had a door-to-balloon time of 75 minutes, vs. 684 minutes for the 25 STEMI(-) OMI with pre-cath opioids.
= = =
======================================
MY Comment, by KEN GRAUER, MD (12/30/2025 )
Today’s case by Dr. Nossen features a 79-year old woman who was awakened by CP (Chest Pain) and dyspnea. When her CP persisted — this patient contacted her primary care physician, who referred her to the ED (Emergency Department) due to “ST depression” on her office ECG (which unfortunately is not available).
- KEY Point #1: The initial ECG in today’s case ( = ECG #1) — is the initial ECG that was recorded in the ED. It’s important to appreciate that our lack of access to the office ECG (which was done some amount of time before ECG #1) highlights our loss of a “golden opportunity” to make a definitive diagnosis of an acute OMI from the initial ED ECG (thereby potentially reducing the time until activation of the cath lab by many hours!).
- Simply stated — we lack the following: — i) Knowing what the relative severity of this patient’s symptoms were at the time she was seen by her primary care physician? (and at the time that ECG #1 was recorded); — ii) We lack the ability to compare the office ECG with the initial ED ECG to see if there have been “dynamic“ ECG changes between these 2 tracings; — and, iii) We lack the ability to correlate the relative severity of CP with ECG findings in the office ECG and the initial ED ECG that was done “X” number of minutes (hours?) after the office ECG was done (ie, reduction or increase in CP severity in association with improved or worsening “ST depression” could have provided KEY correlation data regarding acuity and status of the “culprit” vessel [ie, open or closed]).
= = =
- KEY Point #2: In addition to the important Learning Points put forth by Dr. Nossen — I’d emphasize that in this high-risk patient (given older age; recent history of intermittent CP — with very worrisome history that prompted her ED visit, of being awakened by CP and dyspnea) — a repeat ECG in the ED should have been done within no more than 15-30 minutes after ECG #1 (instead of waiting until the next morning).
= = =
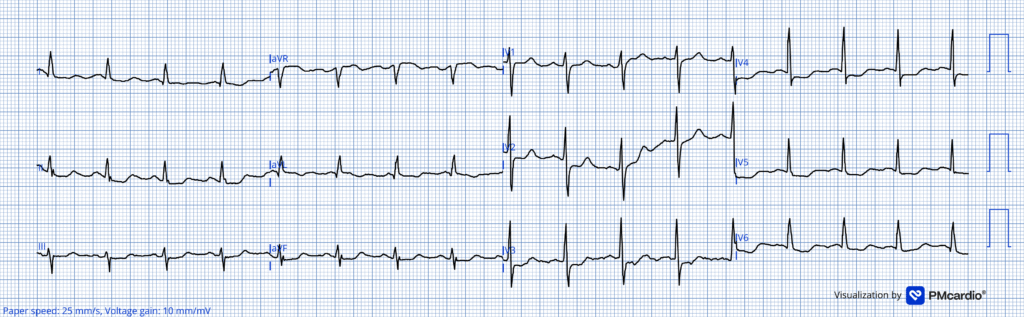
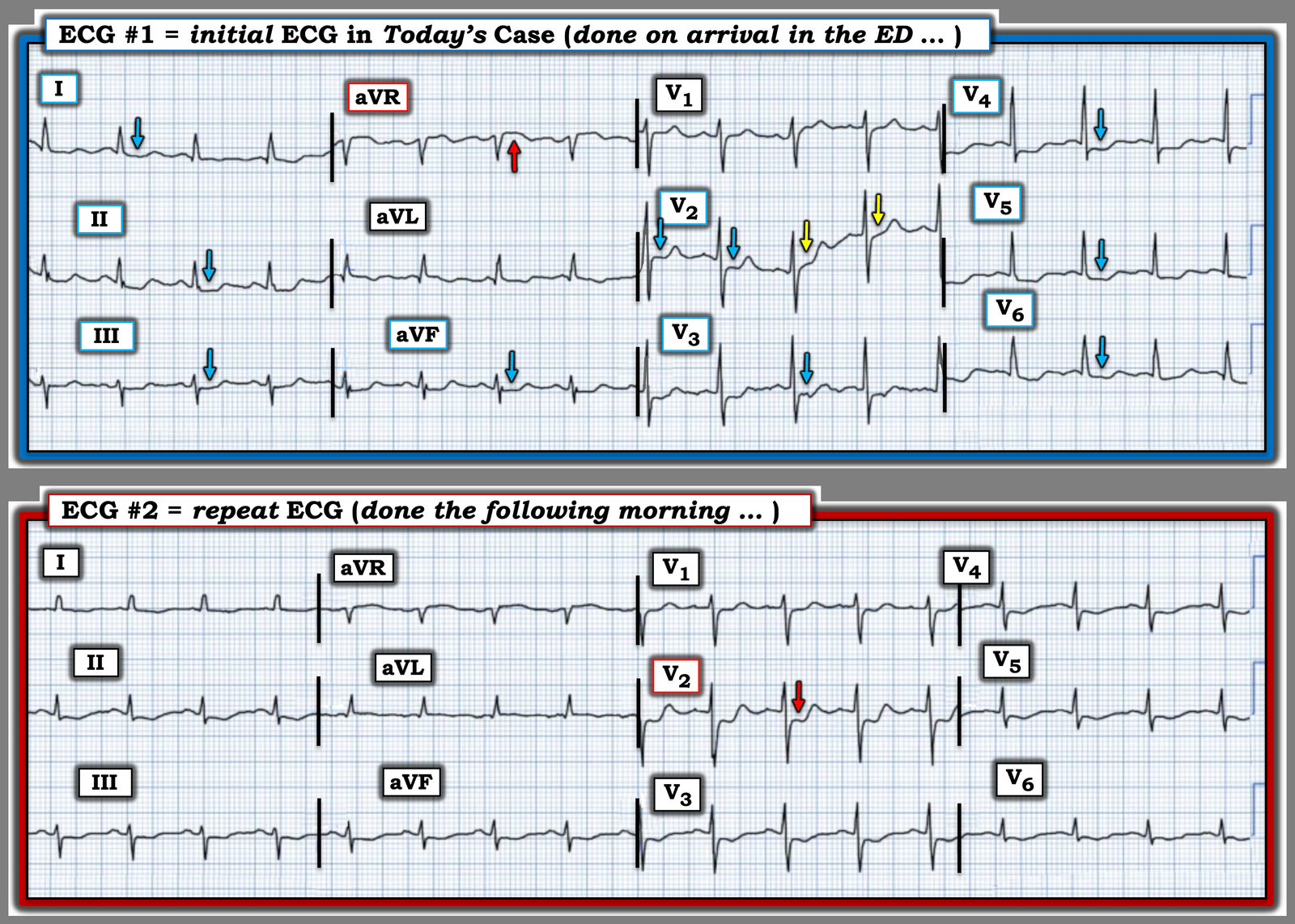
For clarity and ease of comparison in Figure-1 — I’ve put the first 2 ECGs in today’s case together.
- As per Dr. Nossen — the initial ED ECG manifests diffuse ST depression in 9/12 leads (BLUE arrows in leads I,II,III; aVF; V3-thru-V6 — and almost certainly also in lead V2 given the first 2 BLUE arrows in this lead) — in association with ST elevation in lead aVR (RED arrow in this lead).
As emphasized on many occasions in Dr. Smith’s ECG Blog (See My Comment in the March 1, 2023 post, among many others) — this ECG pattern that we see in ECG #1 strongly suggests DSI (Diffuse Subendocardial Ischemia = SEI [SubEndocardial Ischemia]) — and should immediately prompt the following diagnostic considerations:
- Severe Coronary Disease (due to LMain, proximal LAD, and/or severe 2- or 3-vessel disease) — which in the right clinical context may indicate ACS (Acute Coronary Syndrome).
- Subendocardial Ischemia from another Cause (ie, sustained tachycardia — sinus or from some other arrhythmia; shock/profound hypotension; GI bleeding; anemia; etc.).
NOTE #1: Given the history in today’s case (being awakened by sudden onset of severe CP) — DSI in a patient with severe coronary disease has to rise to the top of our list of diagnostic considerations.
- NOTE #2: As has also often been emphasized in Dr. Smith’s ECG Blog — the rapid heart rate in ECG #1 (almost 100/minute) in association with the dyspnea this patient awoke with, should suggest another of the causes listed above (in addition to severe underlying coronary disease) as the reason for her symptoms and the ECG findings in this initial tracing.
= = =
Take another LOOK at both ECGs shown in Figure-1:
- QUESTION: What difference(s) do YOU see?
= = =
Figure-1: Comparison between the initial ED ECG — and the repeat ECG done the following morning.
= = =
ANSWER: What difference(s) do YOU See?
KEY Point #3: Note how much EASIER it is to identify differences between the 2 tracings in Figure-1 — when you compare lead-by-lead with both ECGs right next to each other!
- As per Dr. Nossen — the RED arrow in lead V2 of ECG #2 highlights the obvious change in shape, as well as the increase in ST depression seen in this repeat ECG compared to the initial ECG.
- In addition — I thought there is also less marked ST depression in most of the leads in ECG #2 compared to ECG #1 (including less ST elevation in lead aVR!). To me — this ECG picture in the repeat ECG now clearly suggests both severe underlying multi-vessel coronary disease and acute posterior OMI (given what now clearly is maximal ST depression in lead V2! ).
- KEY Point #4: Given the above findings in ECG #2 — I have to wonder if ECG #1 would have also been diagnostic of acute posterior OMI had the initial tracing been immediately repeated to obtain an isoelectric baseline! (ie, the 2nd BLUE arrow in lead V2 of ECG #1 suggests that there was maximal ST depression in this lead on the initial ECG — and — the wandering baseline in lead V2 invalidates the YELLOW arrow ST segments from our consideration). Had the initial ECG been repeated within 15-to-30 minutes with attention to obtaining a level baseline — a definitive diagnosis might have been reached hours earlier than what occurred.
= = =
My Final KEY Point: Although Dr. Nossen already included my final point among his Learning Points — it bears repeating. That is, the presence of severe underlying, multi-vessel coronary disease will often complicate discrimination between DSI vs acute OMI vs both DSI + acute OMI.
- In such cases — extra attention to additional investigative modalities may be instrumental for expediting recognition of those patients with both DSI + acute OMI (ie, Frequent serial ECGs looking for “dynamic” ST-T wave changes — Echo showing a localized wall motion abnormality [as Echo did in today’s case] — correlation with serial Troponins and relative severity of the patient’s symptoms with respect to each of the serial ECGs recorded).
= = =
= = =