Don’t forget to watch the Webinar:
Smith and Pendell Meyers interpret ECGs for OMI or not OMI on Monday Feb 12 at 11 AM U.S. Central time. Register here:
https://zoom.us/webinar/
Written by Jesse McLaren
A 65 year old
with a history of atrial flutter, CABG and end-stage renal disease on dialysis presented
with 3 days of fluctuating chest pain, which was ongoing at triage. What do you
think? Do you need posterior leads?
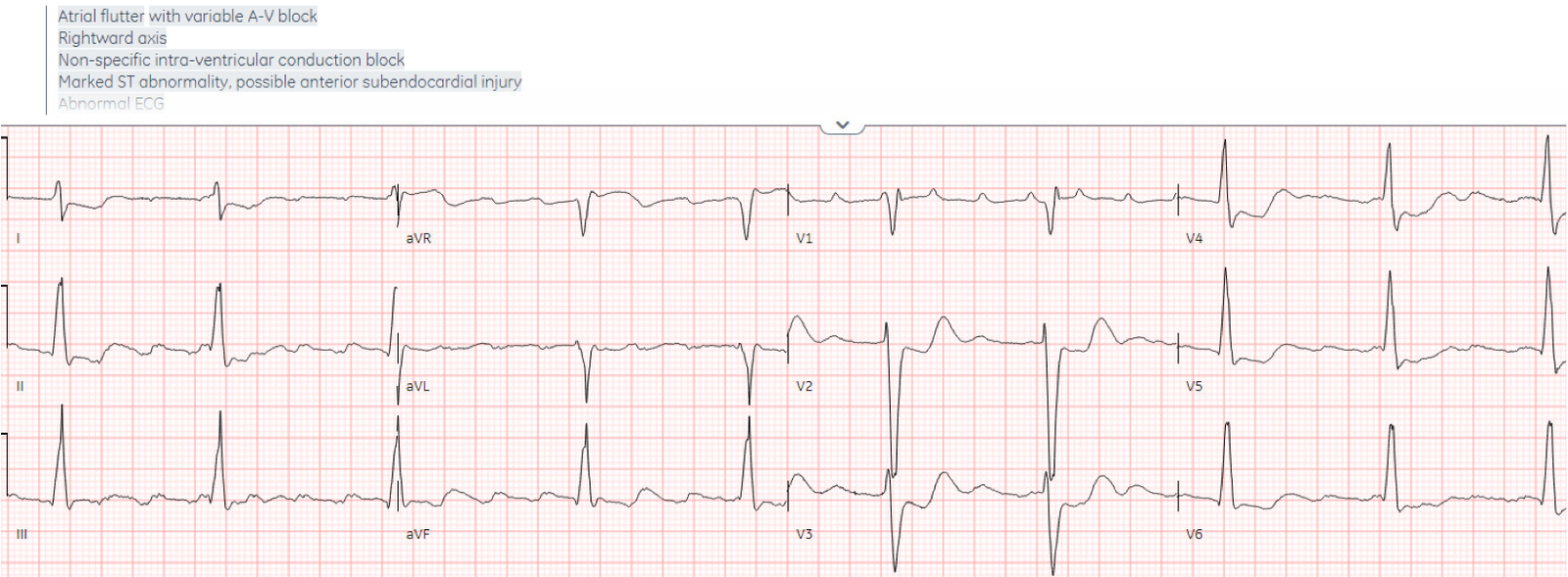
There’s atrial
flutter with controlled ventricular response, a non-specific intra-ventricular
conduction delay, borderline right axis, normal R wave progression and normal
voltages. The abnormal depolarization from the IVCD can produce secondary repolarization abnormalities, but here there appears to be superimposed primary ST depression V2-4 indicating posterior OMI.
Here’s the prior ECG:
This confirms thew anterior ST depression is new. The first ECG was
labeled “anterior subendocardial ischemia”, but subendocardial ischemia does
not localize. If there were diffuse ischemic STD, with precordial STDmaxV5-6
and reciprocal STE-aVR, this would be non-specific subendocardial ischemia from
ACS or supply-demand mismatch. But here there is ischemic STDmaxV1-4, which is not
“anterior subendocardial ischemia” but rather reciprocal to posterior OMI.
So a patient
with high pretest probability (prior CABG with new chest pain), had new ECG
changes showing posterior OMI. Do you need posterior leads? If so, how
will they change management?
Posterior leads are unnecessary if
anterior leads are diagnostic
According to the
STEMI paradigm an ECG has to have ST elevation to diagnose acute coronary
occlusion, and if there’s no ST elevation on anterior leads you can look for it
on posterior leads. But for decades we’ve known that you don’t have to have
posterior leads to diagnose posterior MI in the setting of typical LBBB: In both the Original Sgarbossa
criteria, and in the Smith Modified Sgarboss Criteria, criterion 2 only requires concordant STD in one lead of V1-V3 to diagnose
posterior MI, without the need for posterior leads. In this case there was not
typical LBBB, but the principles of concordant STD still apply.
Smith and Meyers
have also shown that ischemic STDmaxV1-4 is 97% specific for posterior OMI,
without the need for posterior leads.
In this case the IVCD complicates the ECG interpretation, but there was clearly new ischemic STDmaxV1-4, which is diagnostic of posterior OMI.
Here’s the first
ECG flipped upside down, which shows concordant ST elevation in V2-4.
Why not just get posterior leads anyway, to confirm?
Posterior leads can be falsely negative
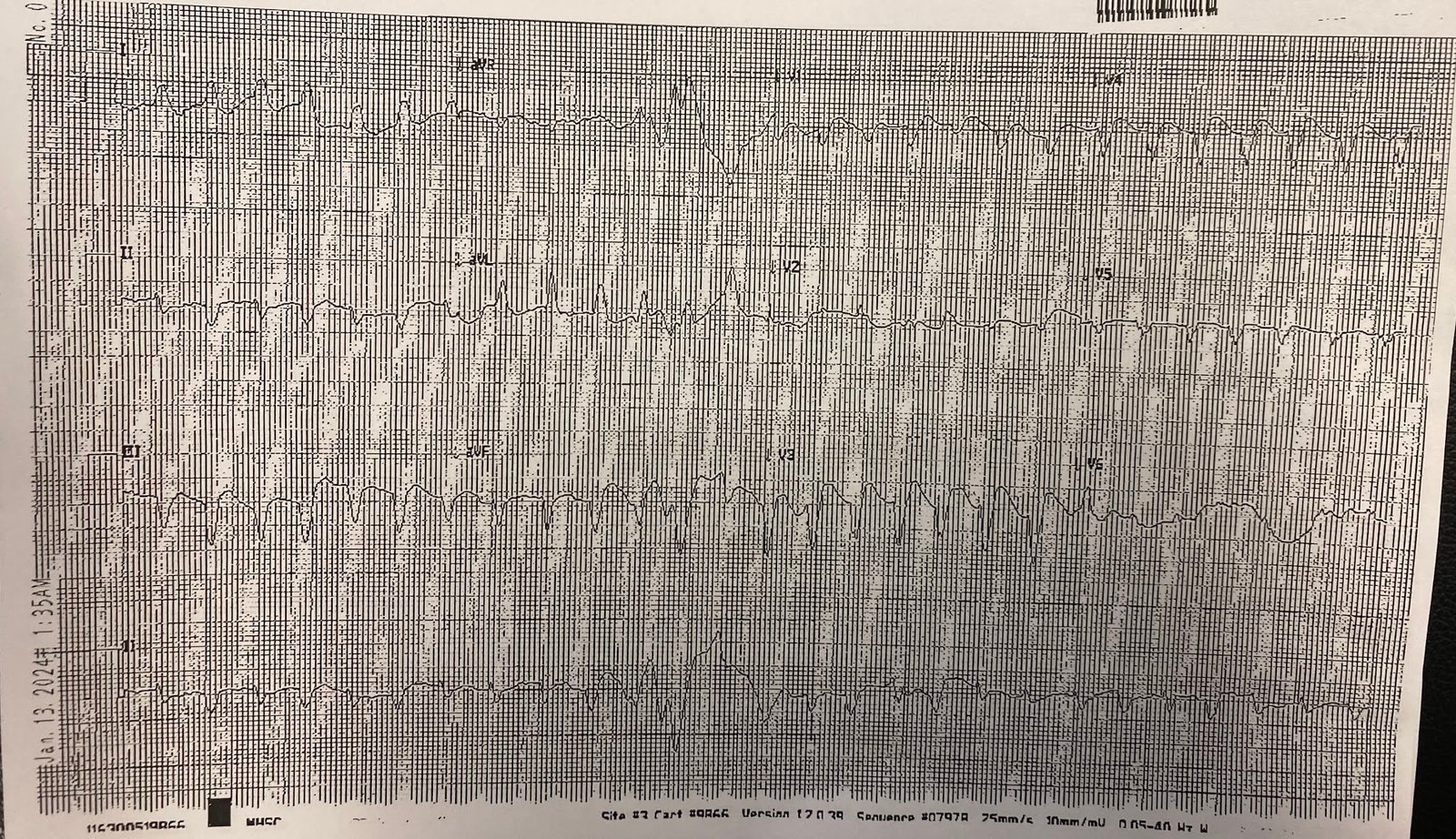
A 15 lead can be
helpful if the 12 lead is non-diagnostic. But if the 12 lead is already
diagnostic the 15 lead posterior leads
can be falsely negative.
The emergency
physician was worried about posterior MI, so recorded a 15 lead ECG:
There’s still
ischemic STDmaxV1-4, but posterior leads are negative. So when the first
troponin returned at 2,200 ng/L (normal <26 in males and <16 in females)
the patient was referred to cardiology as a non-STEMI.
So a patient with
high pretest probability and now lab confirmation of MI, had an ECG with
ischemic STDmaxV1-4 that identified the MI as being occlusive (OMI) rather than
non-occlusive (NOMI). But because there was no ST elevation on either anterior or
posterior leads, they were diagnosed as ‘non-STEMI’ – which can produce diagnostic momentum that
can be difficult to reverse.
‘Non-STEMI’ diagnostic momentum
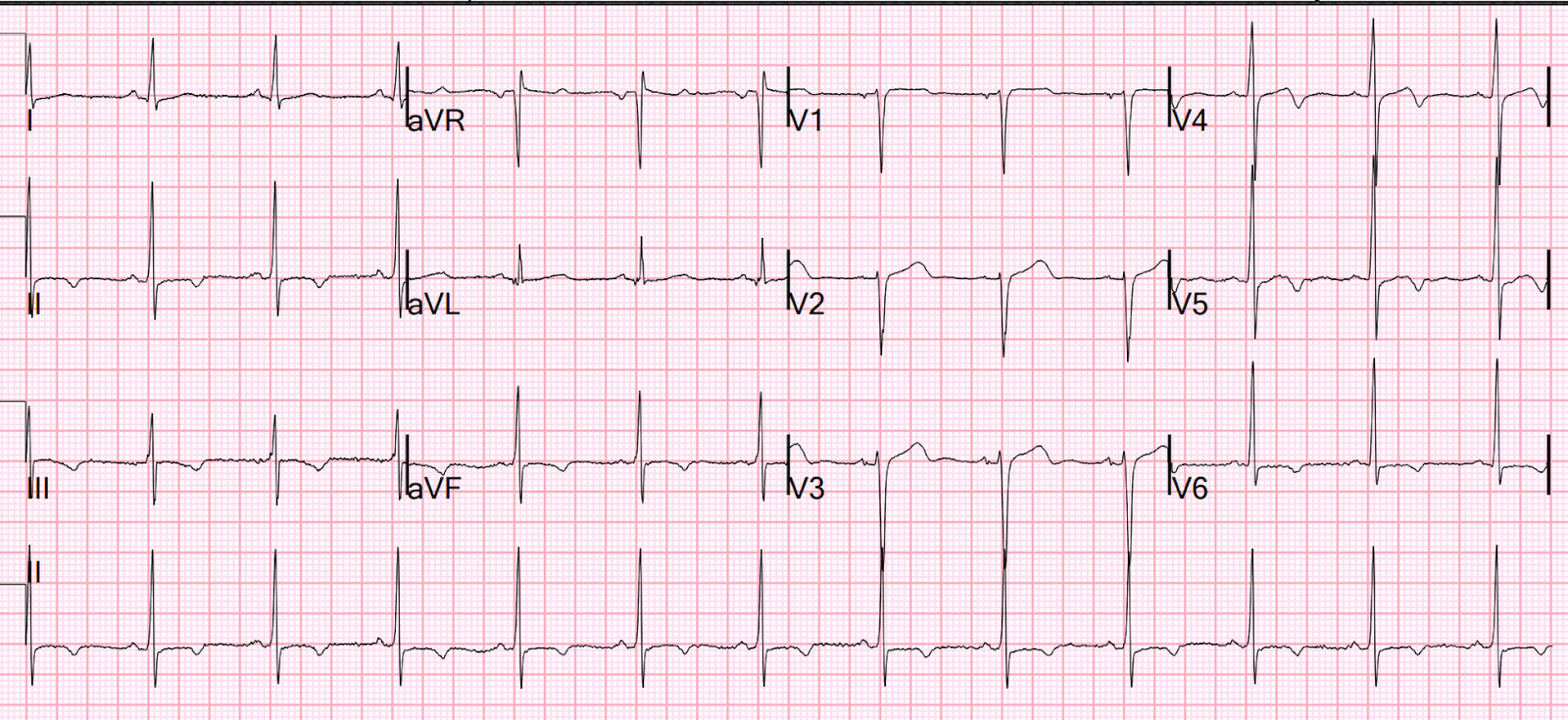
Cardiology
repeated the ECG and troponin, and did a bedside echo.
There’s still
ischemic STDmaxV1-4, but now there’s subtle posterior STE (but still less
obvious than the anterior STD). Cardiology noted “transient concordant ST
elevation”, and echo showed posterior RWMA and CHF with MR.
So now there’s clinical, laboratory, ECG and echo findings of OMI.
But the pain had
improved on nitro infusion and the repeat troponin 2 hours after the first was
the same, so the patient was admitted as ‘non-STEMI’ with a focus on medical
management and dialysis for fluid overload.
The next day
troponin rose to 20,000 and then 50,000, with ECG showing ongoing ischemic
STDmaxV1-4 – which was now interpreted as “anterior STD similar to prior ECGs”:
The following
day the patient had non-urgent angiogram: 95% circumflex occlusion (not fully occluded due to some spontaneous reperfusion in those intervening 20 hours), with peak
troponin >65,000 ng/L (this is a huge infarct).
Discharge
diagnosis was still “non-STEMI” based on the initial ECGs – despite the
diagnostic 12 lead, transient STE on posterior lead, echo findings and massive
troponin elevation.
Discharge ECG
showed normalization of anterior segments.
‘Working diagnosis’ vs ‘final diagnosis’?
The new ESC
guidelines has for the first time merged both STEMI and non-STEMI in the same
guideline because they are both on the spectrum of ACS. They have also
recommended differentiating between the initial “working diagnosis” of STEMI vs
non-STEMI vs the “final diagnosis” based on troponin, echo and angio.
This is a helpful starting point to separate the initial tests from the actual patient outcome. But as an analysis
by Dr. Robert Herman explained, “Although coronary angiography and further
diagnostic testing establish the presence of an occlusive or flow-limiting
lesion as a culprit for the present symptoms, the guidelines continue to give a ‘final diagnosis’ based on inaccurate ECG terminology (ST-elevation and
Non-ST-Elevation Myocardial Infarction). This reinforces the logical fallacy of
the STEMI vs. NSTEMI paradigm called the ‘No False Negative Paradox,’ in which
no NSTEMI patient can ever be recognized as a false negative for OMI,
regardless of their underlying pathology or their benefit from emergent
reperfusion.”
In this case all
investigations – ECG, echo, peak trop, and angio – confirmed OMI, but the
‘working diagnosis’ never changed. But the very first test could have
identified OMI at triage, before the first troponin was back, and reduced
reperfusion delay by two days.
I sent the first ECG to Smith and Meyers without any clinical information or comparison to prior ECG, and they both immediately identified posterior OMI.
The Queen of Hearts had the same interpretation:
YOU TOO CAN HAVE THE PM Cardio AI BOT!! (THE PM CARDIO OMI AI APP)
If you want this bot to help you make the early diagnosis of OMI and save your patient and his/her myocardium, you can sign up to get an early beta version of the bot here.
This is for the version housed on Telegram:
https://share-eu1.hsforms.com/18cAH0ZK0RoiVG3RjC5dYdwfyfsg
You can get the full PM Cardio app here if you live in the UK or EU (or say you do upon registration):
Take home
1. Ischemic STDmaxV1-4 is highly specific for
posterior OMI, without the need for posterior leads.
2. Posterior leads can be falsely negative and lead to missing an OMI
3.
‘NSTEMI’ triaged for non-urgent angio should be reconsidered if
there is dynamic ST changes, refractory ischemic, large troponin elevation, or
echo findings of RWMA
4. To differentiate between ‘working’ and ‘final
diagnosis’ of ACS we need to shift to OMI paradigm to acknowledge missed occlusions retrospectively and work to identify them prospectively
5.
Queen of Hearts can identify STEMI(-)OMI